ResourceSeparations
Robustness of RapiFluor-MS N-Glycan Sample Preparations and Glycan BEH Amide HILIC Chromatographic Separations
Robustness of RapiFluor-MS N-Glycan Sample Preparations and Glycan BEH Amide HILIC Chromatographic Separations
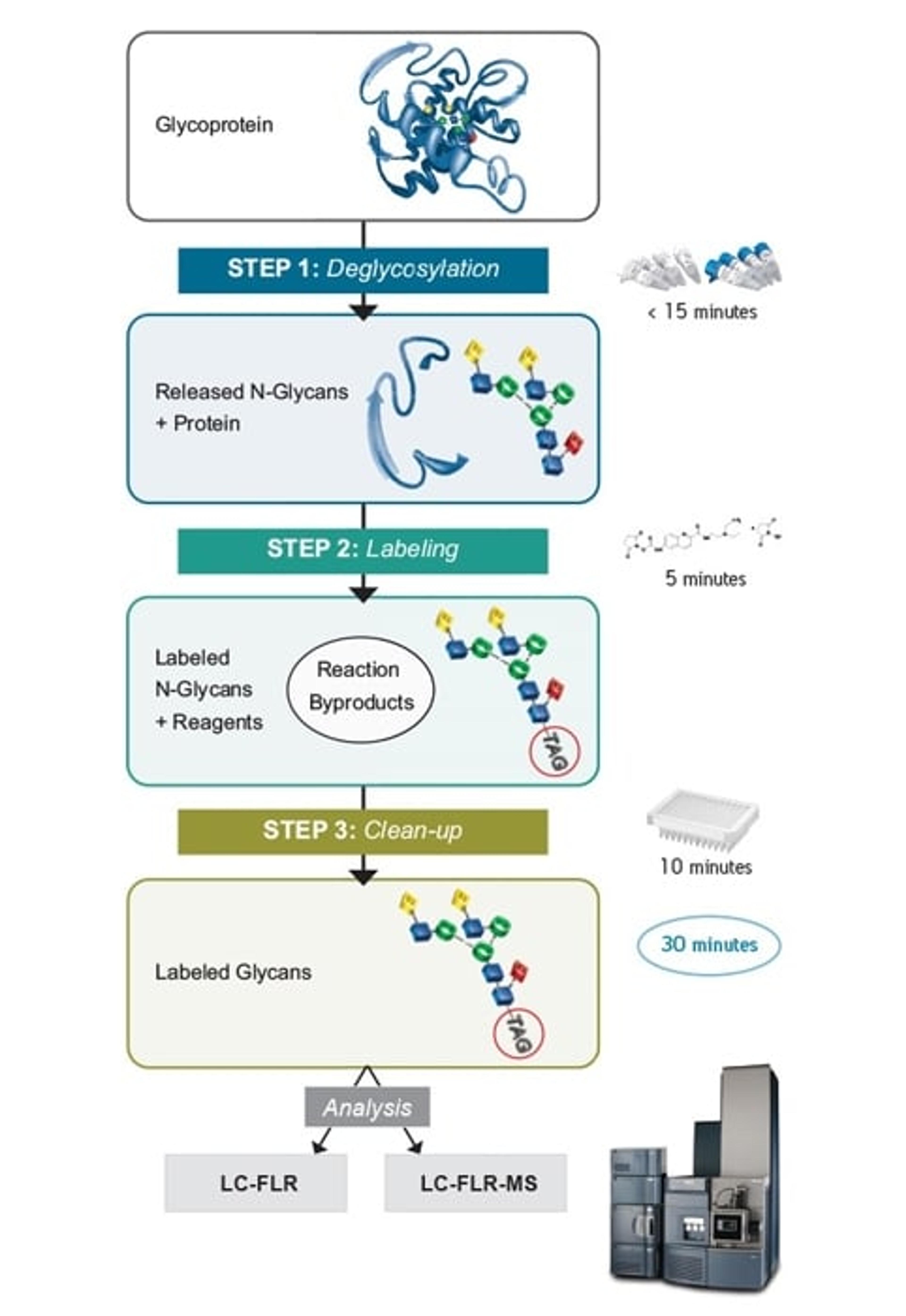
11 Jan 2016N-glycosylation of proteins is routinely characterized and monitored because of its significance to the detection of disease states and the manufacturing of biopharmaceuticals. Glycosylation profiles are most often assessed by means of released glycan analyses, wherein samples are often prepared by techniques that are notoriously time-consuming or lead to compromises in MS sensitivity. This application note discusses the attributes of RapiFluor-MS based sample preparation and the corresponding HILIC-based LC analyses.