Simplifying the journey from molecular diagnostic assay development to commercialization
30 Jul 2024
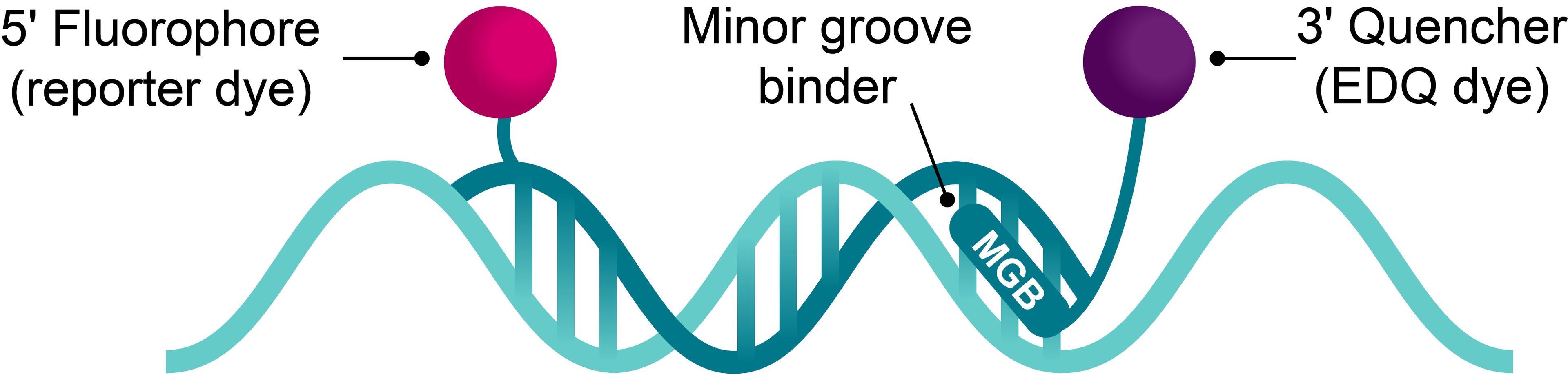
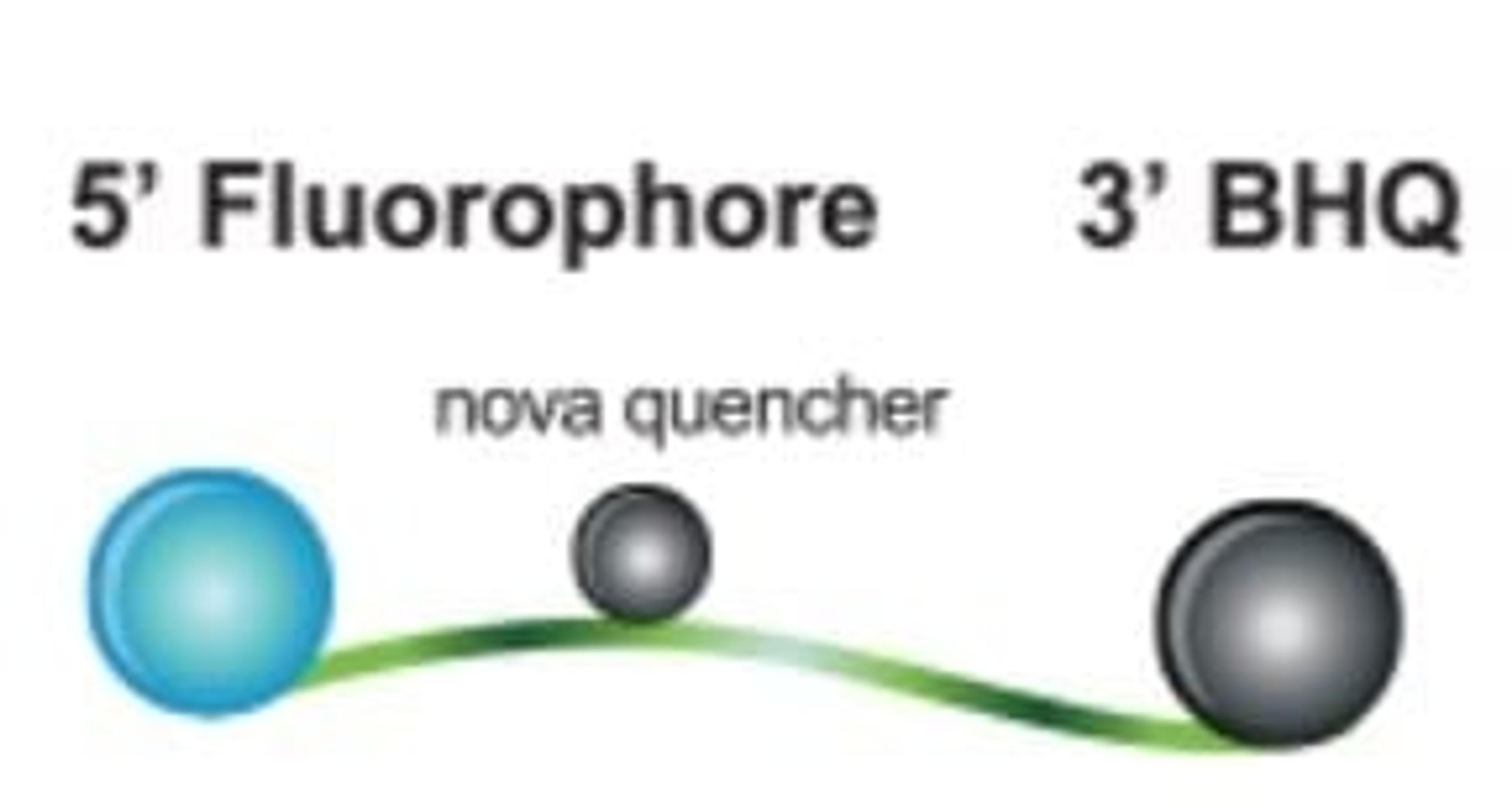
Molecular diagnostics (MDx) is rapidly advancing, with a growing array of DNA- and RNA-based tests now available to physicians, pharmacists, geneticists, research scientists, and other healthcare professionals.
As labs work with human and microbial genes, test developers must comply with clinical guidelines and regulations. Both established companies and start-ups face challenges, including meeting regulatory standards, ensuring quality, and managing supply chain issues. Early planning and robust partnerships are key to overcoming these obstacles.
This essential guide explores these challenges through detailed case studies. It covers everything from early development and assay formulation, to supply chain security, offering expert insights on successfully bringing PCR, next-generation sequencing (NGS), and other nucleic acid-based diagnostic tests to market.