Product NewsClinical Diagnostics
Revolutionizing CGM with FreeStyle Libre
18 Feb 2019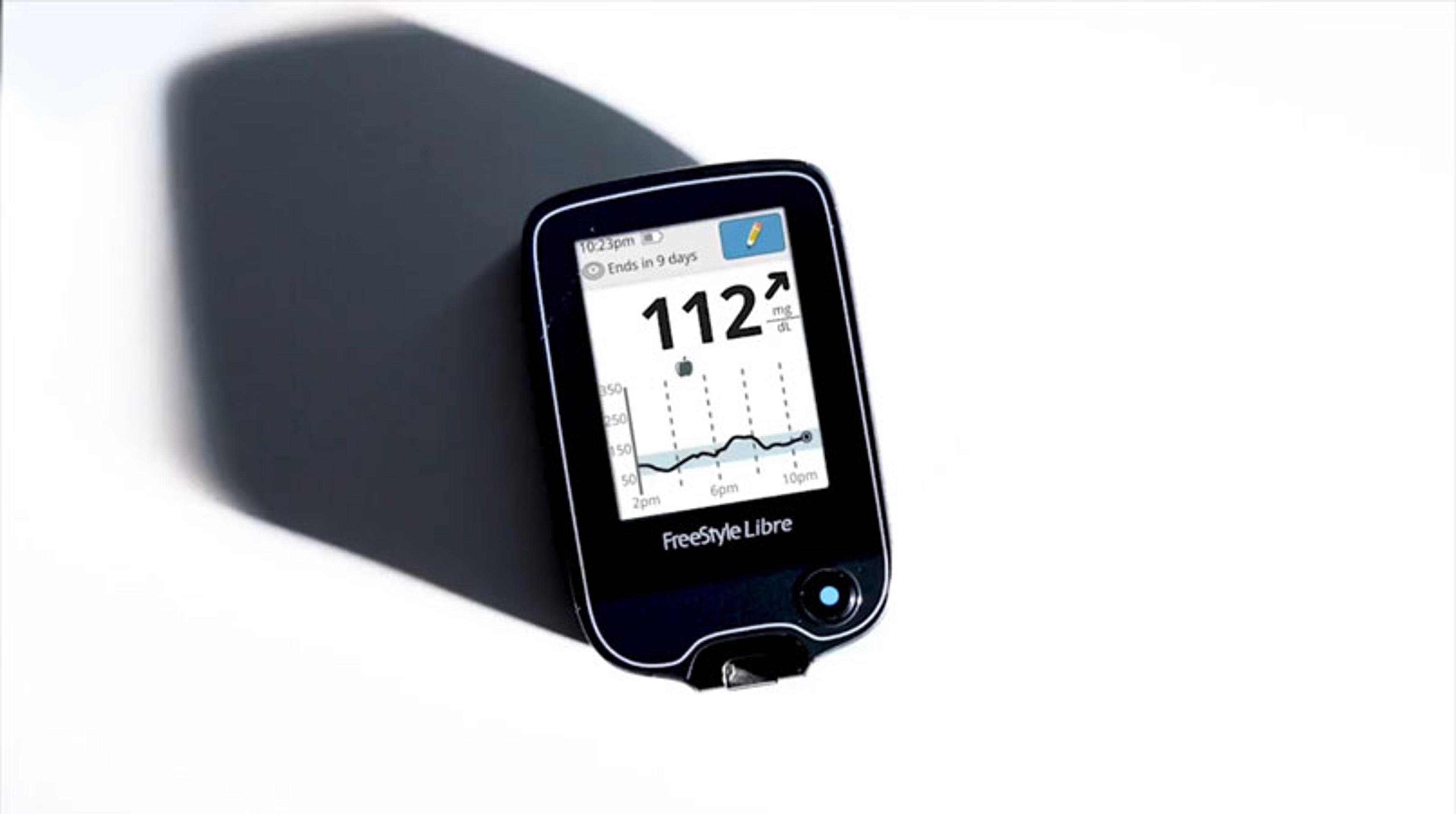
This video presents a guide to the FreeStyle® Libre from Abbott, which has recently been approved by the U.S. Food and Drug Administration. For the 30.3 million Americans who have diabetes, FDA approval is a potentially life-changing experience. This flash glucose monitoring system aims to replace blood glucose testing and is used for detecting trends and patterns in order to help recognize episodes of hyper- and hypo-glycemia, facilitating insulin therapy adjustments. The revolutionary system contains a factory pre-set calibration and therefore eliminates the hurdles of traditional glucose monitoring and requires no routine fingersticks or fingerstick calibrations.

