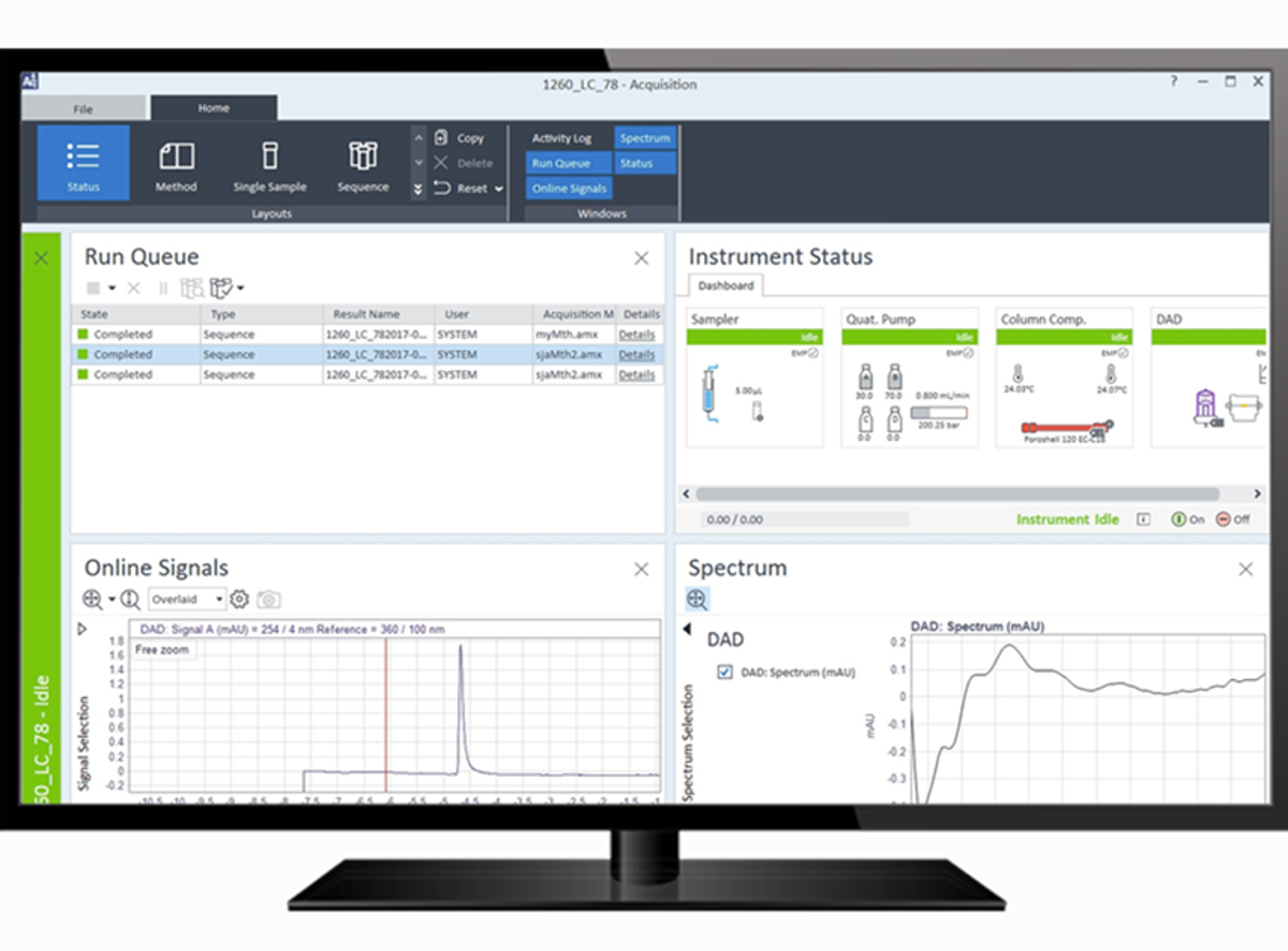
Eliminate errors with automation and stress-free sample tracking
In a typical laboratory, quality control mishaps are inevitable during sample preparation. For example, a sample going missing or a sample being miss-identified will result in downstream effects including the loss of significant time, money and samples. Therefore, advances in digitalization have been shown to reduce human error as manual input and transcription is eliminated from the process. Consequently, this lowers the likelihood of obstacles such as these, to improve data accuracy and process efficiency.
Join Dr. Kelsey Mato, Product Marketing at Agilent Technologies, to hear about a new technology, linking samples and software for unprecedented control, to give you confidence in your laboratory workflow.
Key learning objectives:
- Understand the long-term risks and costs of manual sample tracking
- Learn how taking steps toward digitalization can reduce errors in sample management
- Find a new solution for seamlessly linking samples and software
Who should attend?
Lab managers, and experienced and inexperienced lab technicians.
Certificate of attendance
All webinar participants can request a certificate of attendance, including a learning outcomes summary, for continuing education purposes.
Speakers

Dr. Kelsey Mato completed her PhD in analytical chemistry at Louisiana State University. Her career began focusing on sensing devices in the biopharmaceutical industry as well as her involvement in software for a wide range of instruments. Ensuing this propelled her efforts to specialize in analytical instrumentation where currently she is working as a product marketing professional at Agilent Technologies.
Moderator