5 essential resources to streamline your drug discovery and development processes
Explore the latest application eBooks with top tips on biopharmaceutical characterization and high-content screening
7 Jan 2022
In this article, we have compiled the latest free application eBooks with all the information you need to improve your drug discovery and development processes. Read on to discover how to achieve deeper characterization of biotherapeutics and explore the latest trends in immunoassay technology. Plus, gain insight into how to successfully develop methods for antibody-based therapeutics and more.
Biopharmaceutical characterization: Improve predictive screening
Due to their large size and complex structures, characterizing biotherapeutics requires robust and demanding analytical techniques. In this eBook, discover how you can achieve deeper characterization of biotherapeutic proteins using light scattering techniques.
Download guide>>
Your guide to miniaturized immunoassays for biotherapeutics
Explore the advantages of automated, miniaturized immunoassays across the drug development pathway, from pre-clinical to bioprocessing, and discover how automated, miniaturized immunoassays can help you maximize productivity.
Download guide>>
High-content imaging for diverse 3D cell culture models
Learn how to capture in-depth and high-quality images of spheroids, stem cells, organs-on-chips, and whole organisms, and read case studies from the scientists developing 3D cell assays to delve into the complex biology of neurodegenerative disease, angiogenesis, and tumor microenvironments.
Download guide>>
Development of next-generation antibodies for therapeutics
The discovery and development of monoclonal antibodies (mAbs) has led to an improved understanding of their therapeutic potential against emerging infectious diseases. In this application guide, we look at some of the successful discovery and development methods for monoclonal antibody-based therapeutics.
Download guide>>
Lab weighing: Achieve compliance with ease
Matching the ever-demanding regulatory needs of the pharmaceutical industry can be challenging and introducing fully compliant instrumentation and protocols into your lab is a huge step in the right direction towards meeting these requirements. Here, explore the importance of weighing compliance in the regulated lab and discover tools to achieve greater lab efficiency.
Download guide>>
Related drug discovery and development resources:
- Navigating the revised European Pharmacopoeia chapter 2.1.7 “Balances for analytical purposes”: Following the release of the revised Ph.Eur., we spoke with Dr. Julian Haller, metrology engineer from Sartorius, who was involved in writing this new chapter, to help answer some frequently asked questions. This guide contains all the need-to-know information on how this update will affect your drug manufacturing processes. Read article>>
- Biophysical characterization of protein reagents: The quality of protein reagents is critical to ensuring reproducibility and consistency for research. In this webinar, Deborah Moore-Lai, director of protein development at Abcam, will introduce a new protein product line and discuss the approach taken at Abcam to ensure batch-to-batch consistency of these proteins. Register now>>
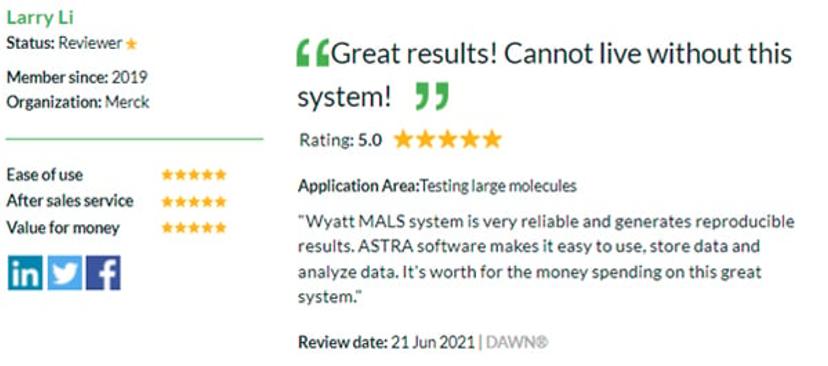
- Shared knowledge: Read reviews left by other drug discovery scientists to help you choose the best instruments for your lab or share your opinion to help your peers by writing your own review today. Read one of our latest reviews below by Larry Li from Merck about the DAWN multi-angle light scattering (MALS) detector.
You can find more drug discovery and development content on SelectScience here >>