7-Fluorophore Method to Detect Rare Cells in Early Stage Cancers
A crosstalk-free 7-plex immunostaining method using widefield fluorescence microscopy shows promise for the detection of early-stage prostate cancer prediction
2 Apr 2019With no cure for cancer, an early diagnosis followed by modern therapy remains a physician’s best bet. A new class of circulating tumor cells shed from a tumor’s own blood vessels right into the patient’s bloodstream shows promise as a biomarker for early detection. But because they are near-identical to normal blood cells, current technologies are unable to detect them in clinical routine. A research team specializing in rare cell detection technology has now found a way to do just that.
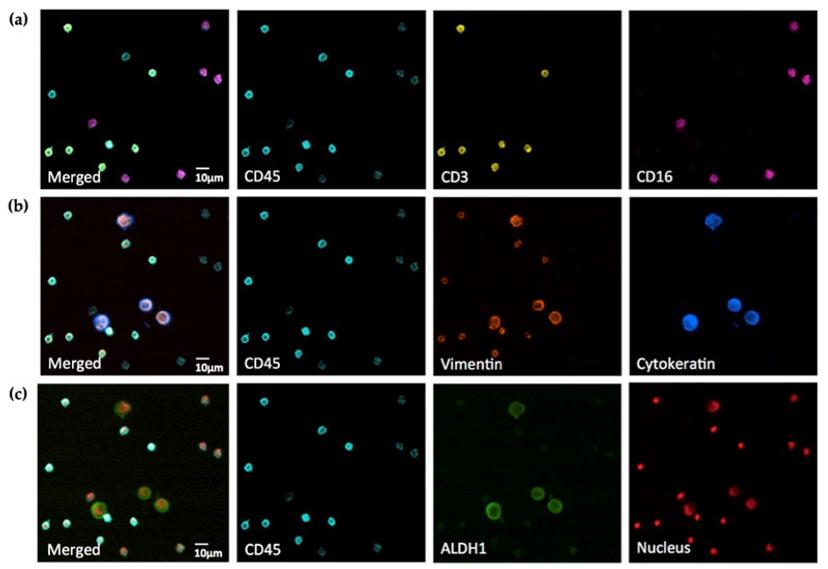

“Our goal has always been to develop clinical protocols and devices that are simple, robust and problem-oriented,” says Dr. Sebastian Bhakdi, CEO and founder of X-ZELL, a biotechnology company whose vision is to reduce the number of deaths caused by undetected cancer to zero. “That ultimately led to the development of a highly multiplexed immunostaining method called Cryoimmunostaining™.”
In this SelectScience® interview, Dr. Bhakdi explains the 7-plex immunostaining process to detect rare cells, the challenges with overcoming crosstalk and the choice of the DNA-staining dye, DRAQ5™.
Headquartered in Singapore and ranked among Asia’s 20 most innovative health technology startups, X-ZELL has developed the new platform technology capable of detecting tumor-derived circulating endothelial cells (tCEC) in a small, 10ml blood sample.
“Finding atypical cells like tCEC in blood is an indication of the presence of cancer,” explains Bhakdi. “It’s a very robust approach because pathology has taught us that atypical cells do not belong to your body unless there's something wrong.”
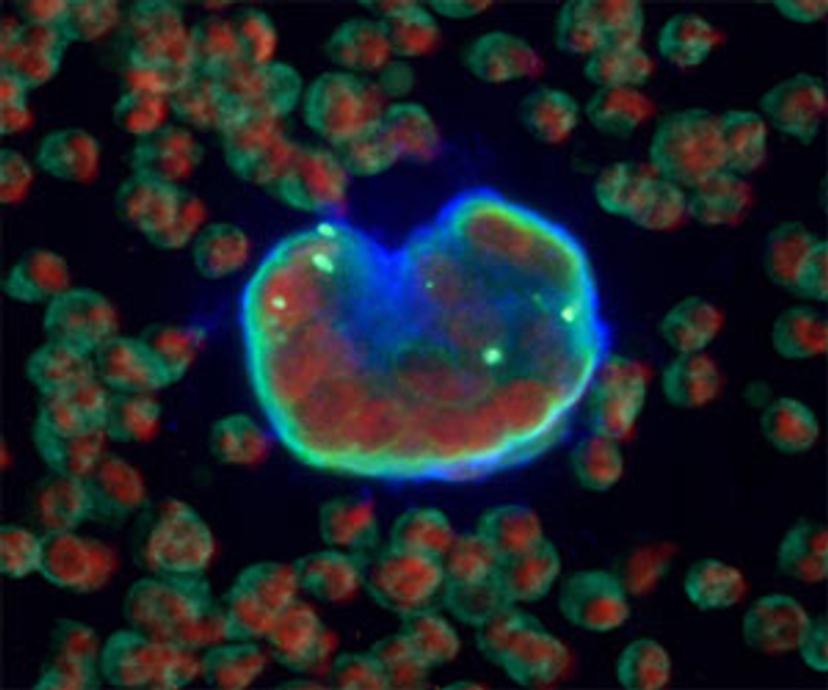
Long considered undetectable in clinical routine, tCEC derive directly from a tumor’s chaotically growing blood vessels and are one of the first types of tumor-derived cells to be shed into the host circulation.
According to Bhakdi, they are not only considered ideal biomarkers for early detection, but also inherently linked to aggressive disease – meaning they provide doctors with a whole new level of information on the nature of the disease.
Because they carry many common blood cell markers and hardly any specific cancer cell surface markers that would distinguish them from normal blood cells, the consensus has long been that they are simply too hard to find in a routine laboratory.
The Cryoimmunostaining™ Method
Bhakdi’s patented Cryoimmunostaining™ method cracked the tCEC code by using a complex panel of fluorophores conjugated with antibodies. The decision to use standard laboratory slides — eliminating the need for expensive equipment or complicated downstream digital processing required for other methods such as confocal microscopy or flow cytometry — comes with one intention: to make this technology clinically relevant, robust and time-efficient.
Instead of applying otherwise expensive and resource-heavy molecular biology methods such as sequencing or proteomics to detect these rare cells, X-ZELL decided to pursue immunocytology “because pathology works all over the world,” says Bhakdi. “The same antibodies work in European, North American, South American, or Asian samples,” making the method scalable across countries.
Detection of 7 Fluorophores: The Challenges
Formulating a 7-fluorophore immunostaining posed technical challenges. “The first challenge was a strong autofluorescence of the cells on the slide where everything lights up without any staining. This wasn’t very helpful as it blocks all the channels,” notes Bhakdi. “Then there was non-specific binding of antibodies. And, of course, the good old crosstalk issue between channels where the spectra overlap.”
Bhakdi’s team addressed each challenge. “We found that if we don’t dry the cells, keep them cool and moist at all times under controlled humidity, they don’t become autofluorescent.”
The nonspecific antibody binding issue was resolved by using coatings on the glass surface that curbed hydrophobic interactions, as well as avoiding any protein denaturing in the protocol. “You also need to pick good quality antibodies,” adds Bhakdi.
The last challenge was eliminating crosstalk between the multiple channels to get seven separate, clear signals.
Crosstalk-Free Detection of Fluorophores
Upon analyzing the absorption and emission spectra of commercially available channels, Bhakdi’s team managed to design a unique set of narrow bandpass filters and employed it in the filter cubes of a widefield microscope to prevent crosstalk between adjacent channels.
The result is a perfectly balanced set-up capable of separating the fluorophores required to isolate and visualize tCEC. “It’s physics at its finest,” says Bhakdi.
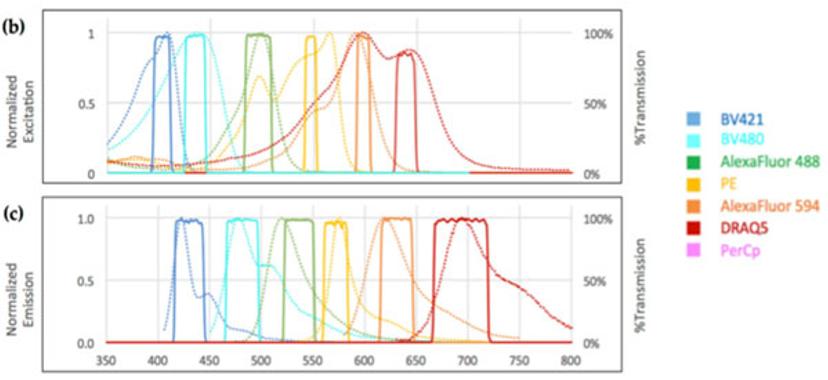
DRAQ5 as the Choice for Nuclear Staining
Part of the tCEC panel is DRAQ5 as the choice for the nuclear staining channel. “Using DRAQ5 frees up two channels in the near-UV range. The DRAQ5 channel overlaps with the Alexa Fluor 647, for which not many antibodies are available,” Bhakdi explains. “If you want to use DAPI, the traditional nuclear stain, it uses up two near-UV channels of the DAPI emission for which there are many good antibodies.”
“So, by using DRAQ5, we actually gain options in our panel and at the same time, we have a very stable DNA dye which just works better than DAPI,” he adds. “Also, DRAQ5 doesn't leach. It stays put on the slide forever. It was a no-brainer for us to try DRAQ5 and incorporate it into our standard panel.”
With over 200 positive reviews by scientists from around the world, DRAQ5 by BioStatus has become the first consumable to win a SelectScience Platinum Seal of Quality.
Predicting Clinically Significant Prostate Cancer
X-ZELL is currently using the Cryoimmunostaining™ method to routinely detect tCEC in patients with clinical suspicion of prostate cancer.
“In our latest study, we enrolled 170 patients who were scheduled for prostate biopsy with suspected prostate cancer due to elevated prostate-specific antigen (PSA), and took their blood immediately before the biopsy,” he explains.
In this blinded study, the results showed that combining PSA values with X-ZELL’s rare cell detection in the diagnostic grey zone nearly doubled PSA’s positive predictive value, thereby leaving less room for false positives, while maintaining a negative predictive value close to 95%, potentially avoiding 7 in 10 unnecessary tissue biopsies. By using its tCEC test as an add-on to the traditional PSA test for prostate cancer, X-ZELL hopes to avoid over-diagnoses triggered by PSA readings in the so-called diagnostic grey zone – ensuring only patients that really need it undergo follow-up invasive procedures.
“We have shown that we can really improve the predictive values of PSA, both the positive value (meaning the patient has cancer) and the negative (meaning the patient doesn't have cancer),” says Bhakdi. “That was only possible because our system not only gives us more room to be creative, but is robust enough to apply it at scale.”
With a goal of expanding the application to other forms of cancer, Bhakdi summarizes X-ZELL’s future outlook: “Our vision is to find cancers early enough to avoid deaths from undetected disease. To get us there as fast as possible, the next step for us will be to make our Cryoimmunostaining™ technologies available to researchers worldwide.” We are making our platform technologies available to everybody now to help us get there as fast as possible.”