A Polyclonal v Monoclonal Approach to FLC Measurement
Session Overview from the 7th International Symposium: Clinical Applications of Free Light Chain and Heavy/Light Chain Analysis
21 May 2015

Dr Robert Lock, Consultant Clinical Scientist, Immunology & Immunogenetics, North Bristol NHS Trust, UK
There were many interesting talks at the recent Clinical Applications of Free Light Chain and Heavy/Light Chain Analysis International Symposium, held in Edinburgh, UK. Dr Robert Lock, Consultant Clinical Scientist, Immunology & Immunogenetics, North Bristol NHS Trust, UK, addressed the delegation on a Polyclonal v Monoclonal Approach to Free Light Chain (FLC) Measurement. His presentation detailed comparisons between the different monoclonal and polyclonal assays available on the market.
Free light chain analysis
Dr Lock began his presentation by highlighting the assays currently available for assessing FLCs in serum. The Binding Site Freelite® and the Siemens N Latex FLC assay are both currently in use; while a third using Luminex technology may be available soon. There are also a number of ELISA assays available that use monoclonal antisera, which were not included in the rest of the presentation.
The focus of Dr Lock’s presentation was on the differences between the Freelite and the N Latex assays. The Binding Site Freelite assay uses sheep polyclonal antibodies, which recognize an enormous variety of free light chain epitopes while the N Latex FLC assay uses monoclonal antibodies that are targeted against a single antigen epitope.
Free light chain complexity
Dr Lock explained that FLCs have an extraordinarily complex and variable range of antigens due to the wide genetic diversity as well as allotypic variations, somatic hypermutation and post-translation modifications; all of which have an effect on epitope availability.
Monoclonal antibodies are targeted against a single FLC antigen epitope; however, given the highly variable nature of FLCs, including the concealment of epitopes, monoclonal antisera may not detect all FLCs. Polyclonal antisera recognize a wide variety of epitopes on the FLC, meaning that you are much more likely to be able to detect these variations.
In addition, there is a long list of factors that affect any assay reaction, including antigen and antibody concentration; antibody titre and avidity; and ionic strength, all of which can be extremely difficult to control.
Lambda FLC display particular complexity, more so that Kappa FLC, due to extensive genetic diversity as well as the effect of polymerization on epitope availability.
Dr Lock highlighted Katzmann’s postulate: “The least one may expect from an assay purportedly measuring serum free light chains, is that it has to identify all light chain myeloma.”
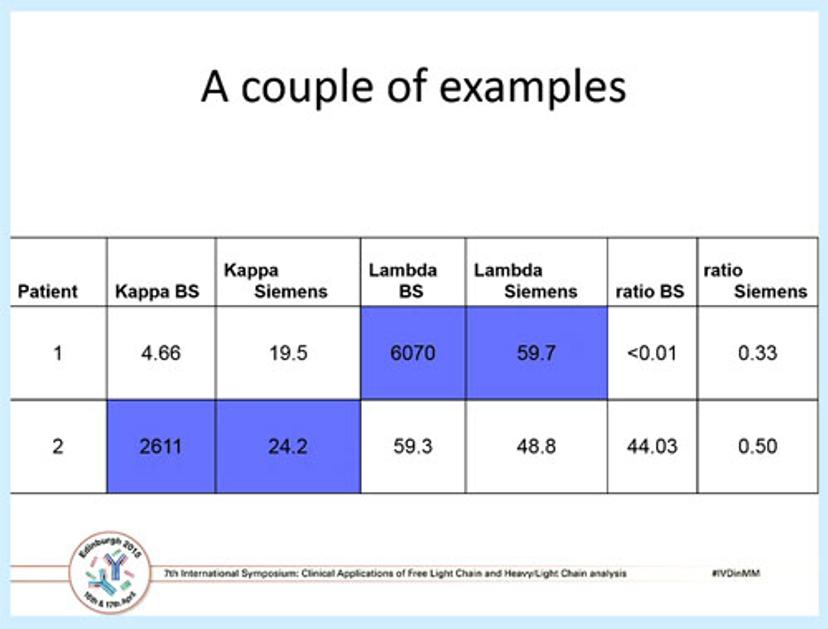
A table showing the comparative results from two myeloma patients, using both the Freelite and the N Latex FLC assay.Dr Robert Lock Bob Lock is a Consultant Clinical Scientist in Immunology and Immunogenetics at Southmead Hospital, Bristol, UK. He has over 35 years' experience in diagnostic immunology.
Dr Lock went on to discuss the results of a multi-centre study he was involved with, which was published a couple of years ago. The above table shows two patients analyzed using both Freelite and N Latex FLC where the kappa and lambda ratios are normal by the N Latex test, but are 100-fold greater and abnormal using the polyclonal Freelite assay. Overall, of the established light chain myelomas in the study 14 patients (5.2%) had clonal FLCs which were poorly detected by the monoclonal antisera. Of these, one case had a highly misleading normal ratio and one case was not detected at all; in addition, more significant outliers were found for lambda than kappa.
It should be noted that these results came from just one study. However, Dr Lock highlighted other examples found in previous studies, including a study by Hoedemakers et al. in 2011, in which it was noted that one patient with lambda light chain myeloma had a normal ratio by N Latex FLC but was abnormal by Freelite. Pretorius et al, 2012, also described three patients with lymphoproliferative disease, where normal FLC ratios were seen in the N Latex FLC assay but all had an abnormal FLC ratio by Freelite.
As a result Dr Lock argued that “Katzmann’s Postulate” would appear not to be met by the N Latex assay. It was his conclusion that some clones might be missed by the more limited epitope specificity offered by monoclonal antisera.
Dr Lock then discussed the equivalency of the two assays, highlighting a number of other studies including one by Di Noto et al. (Ann Clin Biochem. 2015;52:327-36) in which it was stated that “all together, our data highlights that the two methods behave differently in monoclonal samples, particularly at higher concentrations”. Studies by Mollee P et al. (Clinical Chem Lab Med 2013; 51; 2303-2310) and Palladini G et al. Hematol (Oncol 31 (Supplementary 1): 151-200, 2013) also concluded the two assays did not correlate sufficiently to be interchangeable for hematological response criteria. In addition, as many of the international guidelines are based on Binding Site's Freelite assay, he concluded due to these differences they cannot be translated to the Siemens N Latex FLC assay.
In conclusion, Dr Lock asserted that given the data from all of the studies observed, it would seem that the diagnostic sensitivity for the N Latex monoclonal assay isn’t quite there and the assays do not correlate sufficiently to be interchangeable.
View Dr Lock's full presentation here.
