A suite of digital tools to address the bottlenecks in laboratory compliance & asset management
Researchers’ time is heavily taxed by admin and compliance workflows, but digital records and easy access to asset data can help
6 Nov 2024

A recent survey conducted by SelectScience® has revealed the significant demand of admin, compliance, and validation workflows on lab personnel’s time, underscoring the need for streamlined compliance protocols and better visibility of laboratory asset data. The results call for increased use of digital records, unified compliance reports, and holistic asset data analytics – needs that PerkinElmer’s suite of lab workflow solutions, OneSource® Laboratory Solutions, is designed to address.
Time consuming challenges in the lab
The independent survey, conducted on a random selection of laboratory scientists in April 2024, highlighted the significant time burden of administrative, compliance, and validation workflows. Among the key findings, 42% of respondents reported spending over five hours per week on these tasks, while three-quarters indicated they spend more than three hours weekly. Major administrative challenges included data review and compliance, IT management, quality control, asset management, and vendor management. Quality control procedures for lab instruments were identified as particularly time-consuming by the respondents.
The demand for asset visibility and automation
Managing instrument inventory, locations, and service histories is complex, particularly across multiple sites and with multiple vendors. In the survey, 77% of participants expressed a desire for better access to asset data, including location, density, service history, performance, cost, and KPIs. Additionally, 82% emphasized the need for greater visibility into asset usage and productivity. More than two-thirds of respondents reported manually managing their documentation and compliance requirements, with 74% of these individuals indicating a strong need for streamlined compliance protocols.
Asset data, all in one place, with actionable analytics
The survey findings highlight the need for more automated compliance testing protocols and improved methods for collating, storing, and analyzing asset data in laboratory environments. To meet this challenge, PerkinElmer’s OneSource® Laboratory Solutions offers a comprehensive suite of electronic documentation, asset management, and analytical tools designed to help researchers optimize and streamline laboratory operations.
A key component of the OneSource® suite is its Asset Management Portal, a cloud-based application that consolidates instruments across all locations into a single, centralized repository. Assets may be radio-frequency identification (RFID)-tagged, and wireless sensors can automatically capture essential data, including equipment utilization, from both PC-based and non-PC-based instruments, regardless of the manufacturer.
This asset utilization information, along with data on instrument downtime, service history, contract status, age, and analytical method use, is accessible via an online and app-based customer portal. Interactive dashboards as well as customizable analysis tools can then provide a holistic view of asset status, performance, and cost. This helps lab managers uncover valuable insights about their assets that were previously hidden in different data silos and formats. This data can inform key decisions on purchasing, decommissioning, redeploying, and maintaining lab assets, such as whether a fleet should be expanded or downsized, and which instruments require more attention.
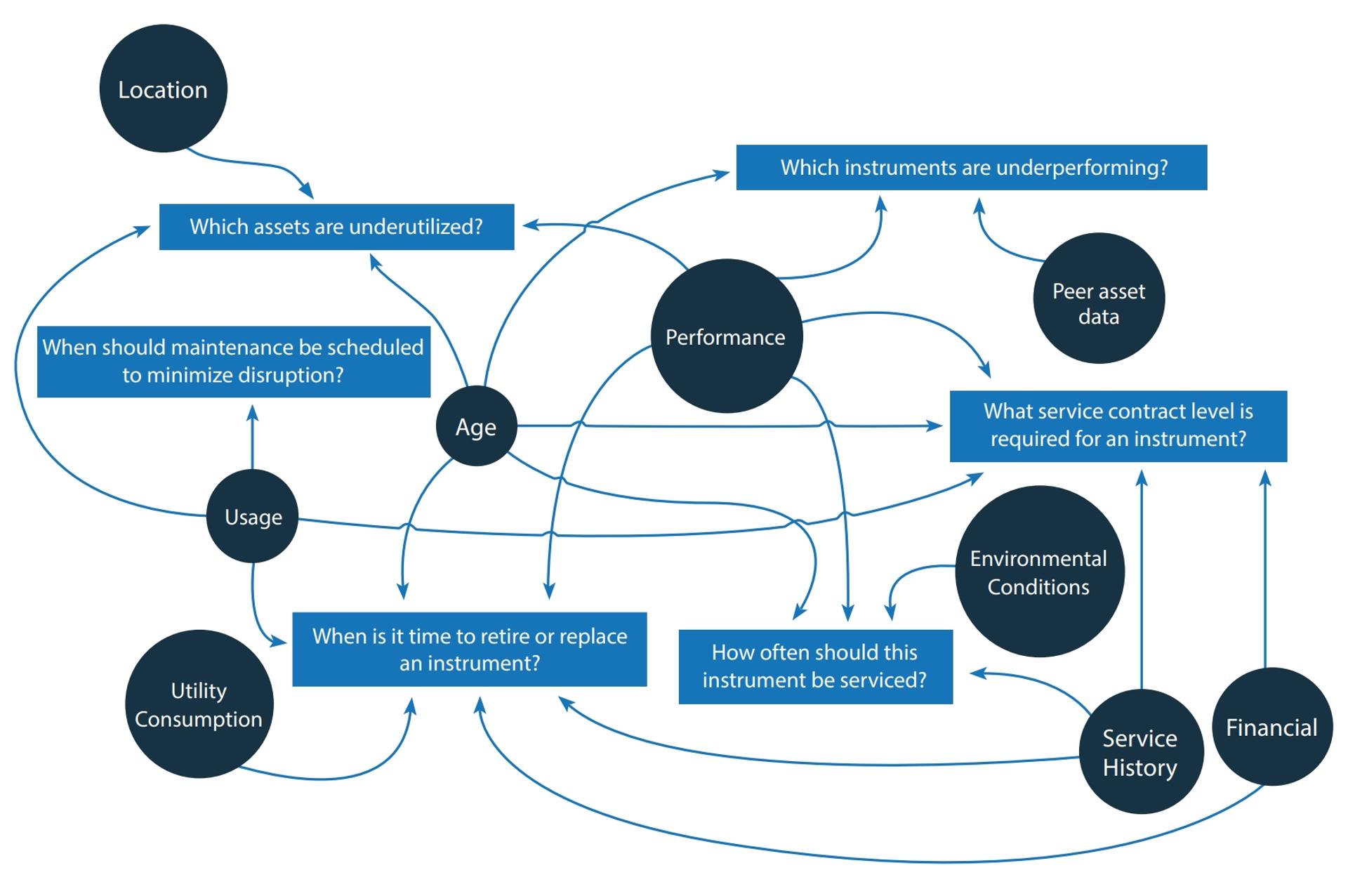
Digitalization and the adoption of connected instruments present labs with vast volumes of asset-related data. By connecting disparate data streams and performing large-scale cross-analyses, PerkinElmer’s OneSource suite can help leverage this data to inform and strengthen lab management decisions.
Streamline compliance testing with universal documentation
In addition to asset utilization and optimization, the OneSource® suite also includes features to help researchers organize and track instrument documentation, which is vital for compliance in regulated industries.
There are various types of compliance testing for laboratory instruments, including installation qualification, operational qualification, performance qualification, and preventative maintenance. This testing not only demands significant time but also extensive record-keeping of equipment purchases, installation, operation, maintenance, test certificates, safety features, and standard operating procedures.
PerkinElmer’s OneSource® Universal Documentation solution simplifies these challenges through an automated process using smart PDFs. The solution streamlines documentation for all major models of laboratory instruments, regardless of manufacturer, producing uniform reports for operational qualification, performance verification, and preventative maintenance. This allows researchers to use the same documents across all brands of the same instrument type, enabling easy performance comparison.
Smart PDFs compile all compliance testing information, including training certificates, test printouts, and raw data, in a single document. Where applicable, they also feature built-in, validated, automated calculations to reduce errors from manual or semi-manual processes. Final PDF reports can be digitally signed and are encrypted with digital certificate technology, preventing modification, deletion, or duplication. They are non-falsifiable and can be safely shared during regulatory audits, complying with 21 CFR Part 11 and EU Commission Annex 11.
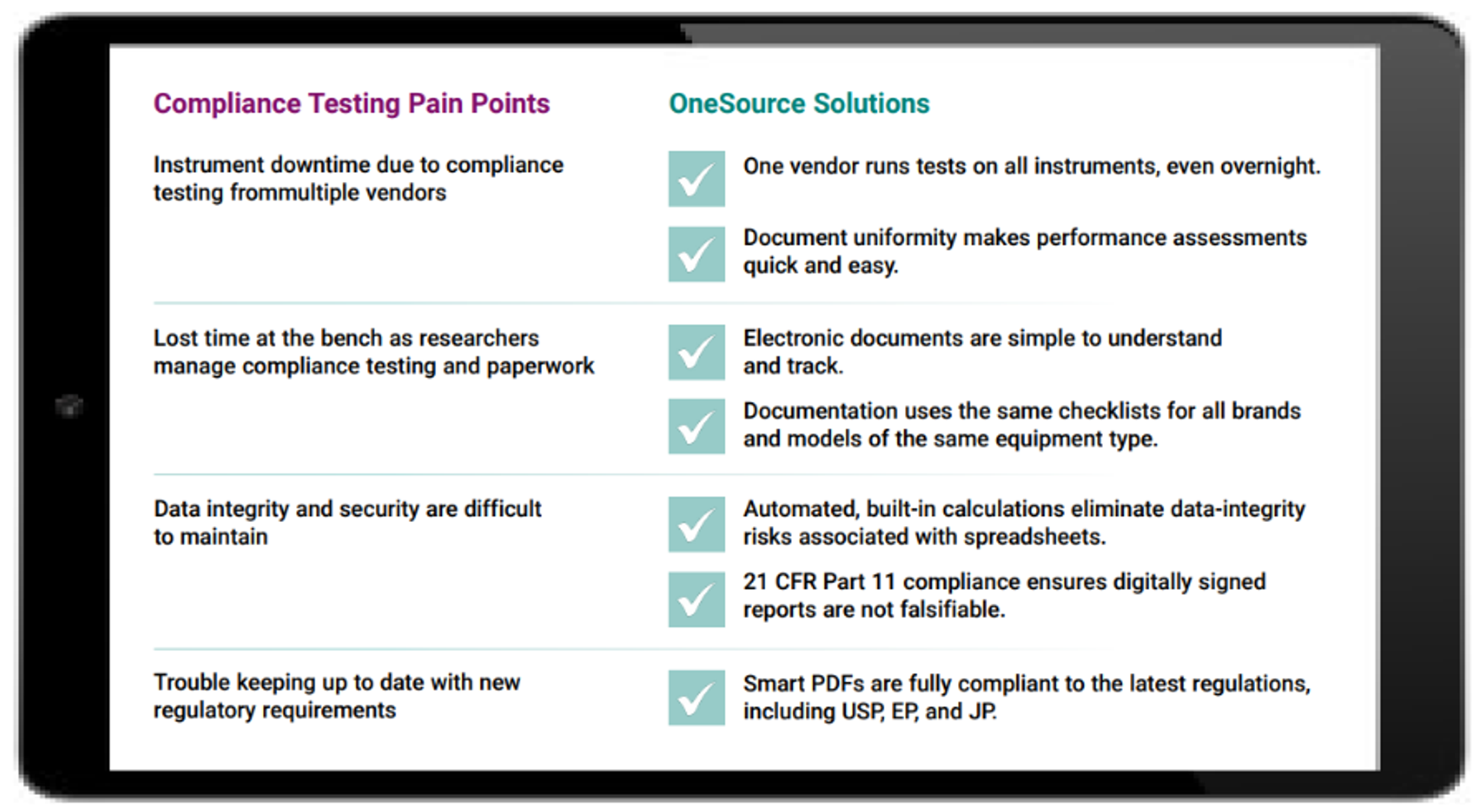
OneSource Universal Documentation replaces the cumbersome paper trail with a simple digital solution, minimizing the risk of noncompliance and regulatory audit while improving lab efficiency.
A single integrated solution
Asset management and compliance documentation are just two elements of PerkinElmer’s OneSource offerings. OneSource offers an end-to-end solution, covering everything from instrumentation and lab IT to compliance, onboarding services, method validation, and general lab support. The service is fully scalable, accommodating both small projects and large managed service programs, with support available remotely or on-site.
The results from the SelectScience survey highlight the urgency in modern labs to free researchers from time-intensive administrative tasks and improve efficiency. By optimizing data management, automating manual processes, and streamlining workflows, solutions like PerkinElmer’s OneSource can empower laboratories to focus on their core scientific pursuits. The potential gain is huge. Spending five hours on administrative tasks each week equates to losing approximately 29 days to admin each year – a full month that could be dedicated to advancing scientific research.

