Automation in liquid biopsy transforms cancer care
New automations in liquid biopsy research hold great promise for bringing us even closer to fully realizing its clinical utility in monitoring cancer from diagnosis through to remission
28 Jun 2024

(Left) Prof. Klaus Pantel, Director of the Institute of Tumor Biology and President of the European Liquid Biopsy Society, and (Right) Dr. Wael Yared, Executive Vice President & Chief Technology Officer at Tecan
Liquid biopsy is rapidly emerging as a revolutionary approach for cancer screening, detection, and as a means of monitoring response to treatment. Unlike traditional tissue biopsies, which require invasive procedures and may not always provide representative samples, liquid biopsies offer a non-invasive alternative that holds great potential of revealing the molecular signature of tumors as well, thereby aiding the individualization of treatments.
The demand for high-quality and scalable research tools and methods in this area is therefore increasing and has pushed leading biomedical manufacturers to introduce innovative solutions for liquid biopsy researchers. Hearing from Prof. Klaus Pantel, Director of the Institute of Tumor Biology and President of the European Liquid Biopsy Society, and Dr. Wael Yared, Executive Vice President & Chief Technology Officer at Tecan, we explore this shift in the liquid biopsy landscape further, and find out how Tecan is working to lead the way in innovation and automation in this exciting space with its pioneering Phase Separator™.
Comprehensive information from a single blood sample
In the last 5 to 10 years, liquid biopsy has seen rapid progress, fueled by advancements in technology and a growing body of clinical evidence. International clinical guidelines have begun to recognize its clinical utility across various disease areas, including breast and lung cancers. These guidelines emphasize the importance of liquid biopsy in early-stage detection and monitoring of disease progression after therapeutic interventions.
Prof. Pantel emphasizes the transformative journey of liquid biopsy, "We have really had great technical advances, particularly in detecting circulating tumor DNA (ctDNA) at very low concentrations, as well as in circulating tumor cells (CTCs) and extracellular vesicles (EVs). There is now the enormous possibility to obtain comprehensive information from a single blood sample."
One of the key drivers of this evolution is the complementary nature of different liquid biopsy approaches. While CTCs provide insights into the physical characteristics of tumors, cell-free nucleic acid techniques, such as ctDNA analysis, offer valuable genetic information. These complementary approaches enrich understanding of disease biology, from fundamental research to patient diagnostics.
Furthermore, recent developments in data science and machine learning have contributed to a deeper understanding of disease dynamics. Dr. Yared highlights the potential of these technologies stating, "It is now possible to model outcomes based on patient histories or to set up digital twins at the population level to assess the impact of screening." These advancements have allowed for more personalized approaches to disease management, with liquid biopsy playing a central role.
A need to make liquid biopsy research scalable
One of the biggest challenges in liquid biopsy research is ensuring the sensitivity and robustness of the assays. Dr. Yared emphasizes the importance of detecting and measuring rare events within background noise, "When analyzing patient liquid biopsies, one is typically looking at the detection and measurement of rare events, in other words a faint signal within a noisy background." Achieving high sensitivity is crucial for accurately detecting biomarkers, such as ctDNA, in blood samples. However, this sensitivity must be balanced with robustness to ensure reproducible results across different samples and laboratories.
Another significant challenge is scaling liquid biopsy research to handle large volumes of samples efficiently. As the demand for liquid biopsy testing grows, laboratories face the daunting task of processing thousands of clinical samples per week. This necessitates automated workflows that can handle high throughput while minimizing error rates and turnaround times.
There is a need for substantial scaling to handle thousands of clinical samples per week, while minimizing error rates, hands-on manual interventions to re-run samples, and overall turnaround times.
Prof. Klaus Pantel President of the European Liquid Biopsy Society
Answering the call to innovate
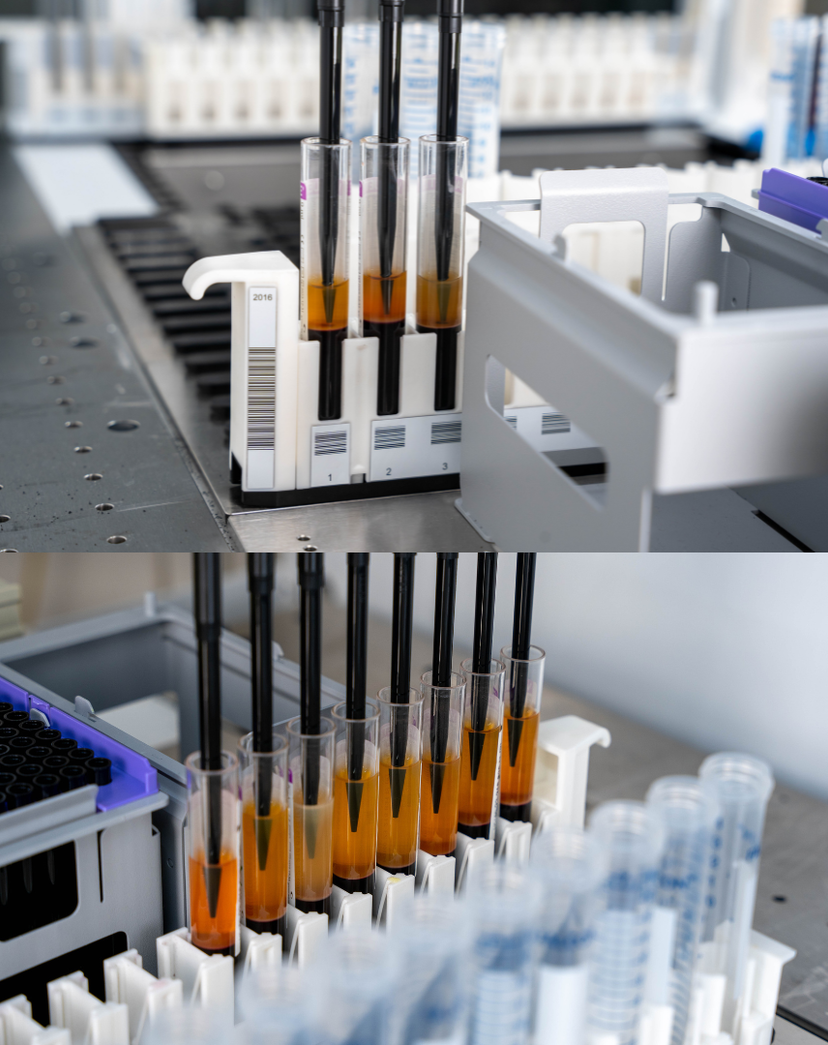
Suitable for removing the plasma phase from a centrifuged blood sample, Phase Separator™ is a unique, pressure-based capability of the pipetting arm. It detects interfaces between liquid layers while aspirating.
Innovative automation solutions will be key in addressing these challenges and driving progress in liquid biopsy research. Dr. Yared expands, "Innovative automation and digitalization approaches are game-changing for our industry. They enable scalability, error reduction, traceability, and decreased turnaround times.”
Recognizing this critical need for automated clinical sample preparation and handling, Tecan launched its unique Phase Separator on the Fluent® liquid handling platform with immediate success and recognition, receiving the Outstanding New Product Award at ISBER2024.
“With Tecan’s Phase Separator, labs can scale at speed while ensuring quality and compliance, empowering their breakthroughs and reducing the total cost of ownership,” Dr. Yared states. “With an impressive speed that is an order of magnitude superior to manual techniques, and it is up to double the speed of conventional camera-based systems, Phase Separator establishes a new standard for efficiency.”
Dr. Yared goes on to share a customer example from a major CDx company, which achieved a near zero error rate with the Phase Separator for plasma separation, significantly outperforming the 15% of previous methods1. By automating liquid biopsy workflows and utilizing Phase Separator, researchers are set to fill a crucial gap in the realm of cancer detection, NIPT, biobanking and other applications.
A future of accessible diagnostics and treatment monitoring
Fueled by continued innovation and technological advancements, the future of liquid biopsies holds considerable promise in delivering a more accessible and comprehensive method of cancer screening, detection, and monitoring response to treatment. As Dr. Yared envisions, "With the benefit of maturing, powerful automation, digitalization, and analysis techniques, we expect liquid biopsy to become a growing part of routine patient care over the coming few years, alongside solid tissue biopsy. Clinical use cases will range from early detection of multiple cancers, to detection of minimum residual disease following primary intervention, to anticipation of disease recurrence and resistance to treatment.”
“We need to disseminate the knowledge that these tests are now available, not only in some expert university centers but really throughout the medical field. Secondly, we need more interventional clinical trials where we use the liquid biopsy to make a clinical decision and then prove that this decision had a benefit for the cancer patient,” adds Prof. Pantel. “For example, for minimal residual disease detection, you can detect a relapse six months or earlier with the ctDNA that's known for colon cancer and for other tumors. But the key question is, what kind of therapy do you then apply, and does it help to apply the therapy six months earlier? These trials have been initiated, and we are eager to see the outputs within the next few years.”
1depending on the liquids and centrifugation conditions.


