Cell-based assays in immuno-oncology: Advancing bispecific antibody development
Explore the importance of robust and reliable cell-based assays in the development of therapeutic bispecific antibodies in immuno-oncology
6 Jun 2023
Dr. Peter Wolff, Implementation Scientist, AGC Biologics
As the field of immuno-oncology continues to expand, researchers are constantly developing new potential treatments to combat cancer. One promising approach is the use of bispecific antibodies (BsAbs), which target specific cells involved in the immune response. However, developing these drugs requires rigorous testing to ensure their safety and efficacy.
Dr. Peter Wolff, Implementation Scientist at AGC Biologics, works to ensure that the company provides the highest quality services by designing, optimizing, and developing cell-based assays for potency release testing together with the company’s quality control department. AGC Biologics is a global Contract Development and Manufacturing Organization (CDMO) specializing in the development and manufacturing of protein-based biologics, and cell and gene therapies, aiming to advance patient care by introducing innovative biopharmaceutical products to the market.
In this article, Dr. Wolff describes the potential of BsAbs as an exciting immuno-oncology therapeutic and the role of the CD3 receptor in their mode of action. Plus, Wolff explores how reliable and robust cell-based assays and analytical techniques are integral in the assistance of bringing these new modality drugs to market.
Bispecific antibodies: Mode of action for immuno-oncology
“BsAbs are a type of therapeutic protein that have tremendous potential for treating various diseases, especially cancer,” Wolff explains. “These engineered antibodies are designed to simultaneously bind to two different target molecules, typically with one antigen present on the surface of cancer cells and the other antigen on immune cells.”
New modality therapies – such as BsAbs – are important for cancer therapeutics because traditional treatments, including chemotherapy and radiation, can have significant systemic side effects and may not be efficacious against all types of cancer. BsAbs offer a more targeted approach to cancer treatment, specifically binding to cancer cells and stimulating a cytotoxic immune response. This makes them potentially more effective and less toxic than traditional treatments and may offer hope for patients who have not responded to other treatments.
“Cancer is notorious for evading the immune system's surveillance by ‘cloaking’ itself from the immune cells, making it difficult for them to identify and destroy cancerous cells,” Wolff explains. “However, BsAbs can effectively remove the cloak and enable the immune cells to recognize and attack the cancer cells.” The potential of BsAbs to direct the immune system towards a particular antigen extends beyond the field of oncology. “Apart from cancer treatment, BsAbs are also being investigated as potential therapies for other diseases such as systemic inflammation and infections,” Wolff continues. “With their ability to target specific cells or tissues, while also engaging the immune system, BsAbs are a promising class of therapeutic proteins that hold great potential for improving patient outcomes in a variety of diseases.”
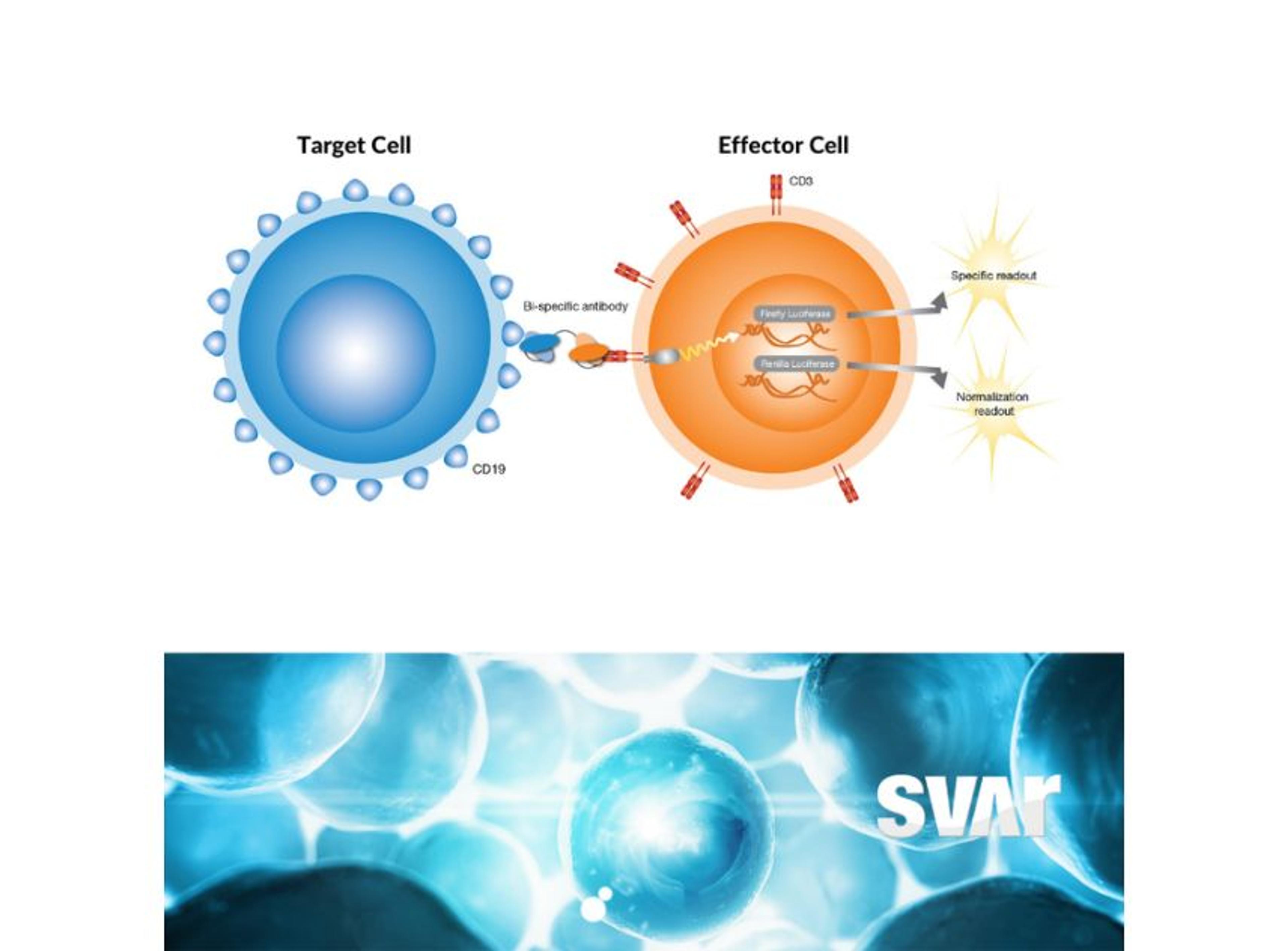
One type of BsAb that has shown promise in cancer treatment is the CD3-engaging BsAb. These antibodies bind to CD3 receptors on T cells and to a specific antigen on cancer cells. By binding to both the T cell and the cancer cell, the BsAb brings the T cell into direct contact with the cancer cell, stimulating an immune response that can help eliminate the cancer cells.
“The CD3 receptor is part of the larger complex of the T cell receptor (TCR) that is involved in antigen recognition and is important in signal transduction for T-lymphocyte activation,” Wolff explains. “The characteristic antigen is expressed on the surface of all mature T cells and helps to transmit signal from the TCR to the interior of the T cell, eventually leading to the desired cytotoxic effect.”
Wolff adds, “by targeting the CD3 antigen and a specific tumor target, BsAbs can redirect T cells and apply the wanted killing effect. One of the key mechanisms of T cell cytotoxicity is the release of molecules like perforin and granzyme, which can induce cell death in the target cell. Perforin creates pores in the membrane of the target cell, while granzyme enters the cell through these pores and triggers the activation of enzymes that lead to cell death.” Wolff continues, “in addition to the release of cytotoxic molecules, activated T cells may also induce apoptosis, or programmed cell death, in the target cancer cell by activating certain signaling pathways. Given their tumor-killing properties, T cells are an attractive target for immuno-oncology therapeutics, and the use of BsAbs is gaining momentum in this field.”
Cell-based assays driving immuno-therapy development
Wolff provides insight into why many new modality drugs require highly precise and accurate assays and analytical techniques during development. “Many new modality drugs are often more highly targeted compared to traditional drugs, and the aim is to achieve a more precise and effective treatment while minimizing side effects. This, of course, will require more specific assays and analytical techniques to investigate the detailed mode-of-action for the drug. A variety of techniques, such as surface plasmon resonance (SPR), ELISA, and characterization assays, are used to assess the structural and physicochemical properties of the novel drug. However, one of the most critical tools for studying the mode-of-action of these drugs in vitro is cell-based assays,” he says.
Cell-based assays are an important tool to measure the effectiveness of potential treatments as they can provide valuable insights into a drug’s mechanism of action and help identify potential issues early in the development process. Identifying issues early in the drug development process is important for ensuring the safety, efficacy, and cost-effectiveness of the drug. “Cell-based assays are used to evaluate how the drug interacts with cells and induces its intended effects,” shares Wolff. “They can provide a more comprehensive understanding of the drug's mechanism of action by simulating the physiological environment in which the drug will be used. This information is critical to ensure the drug's safety and efficacy.”
Cell-based reporter-gene assays
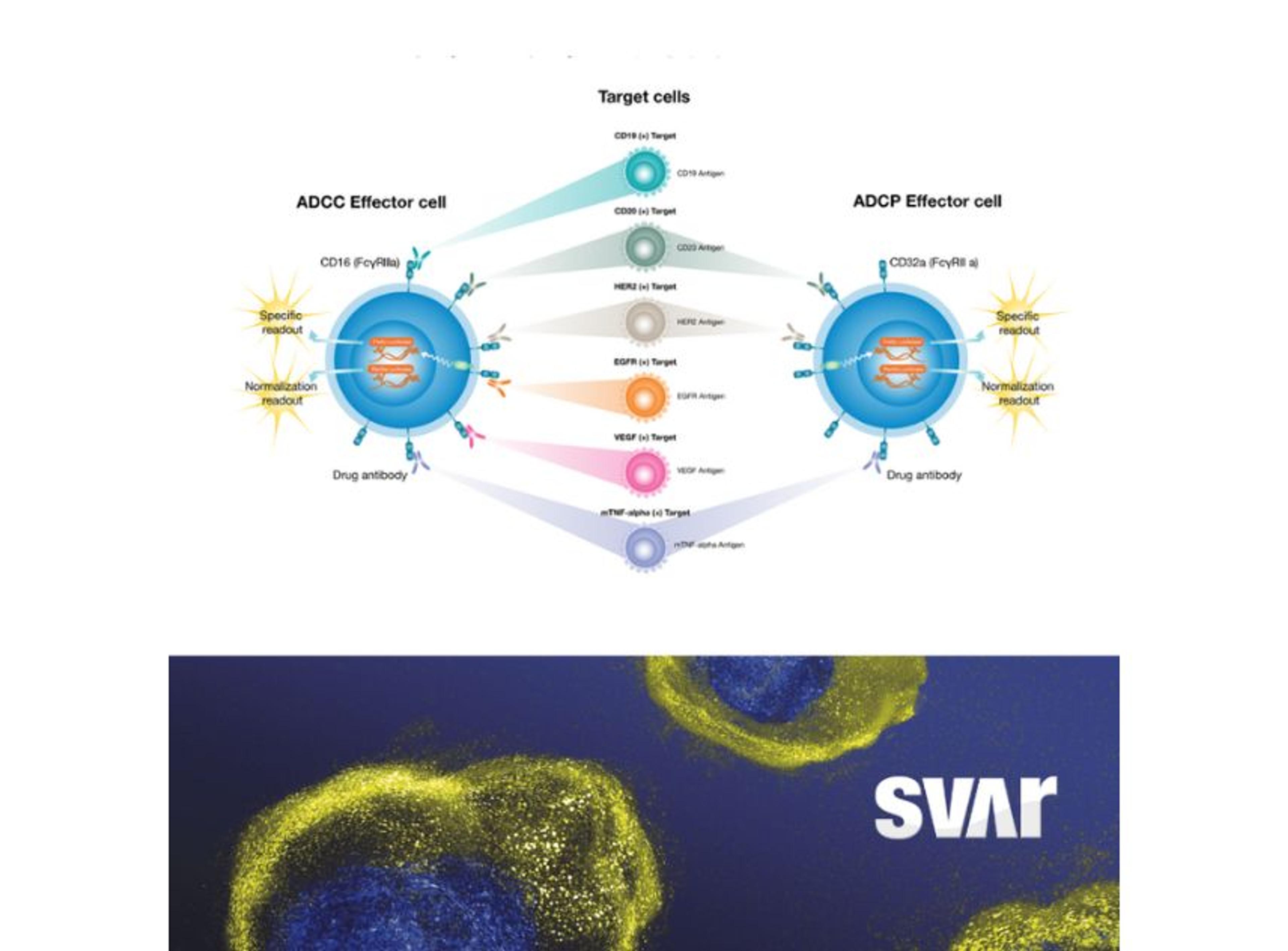
The iLite® CD3 Effector Cells by Svar Life Science are a biologically relevant and powerful tool to assess CD3-induced T-cell activation in the development of novel therapeutic BsAbs. The iLite Target Cells are engineered, homologous, and specific target cells that enable screening for antibody-induced effector functions.
Find out more about theiLite Bioassays >>
Wolff’s team at AGC Biologics effectively utilize cell-based assays in its analytical development work. “Our main objective is to ensure patient safety,” he explains. “To achieve this, we develop, implement, and validate new potency testing methods, which are applied for batch release and to aid in our production.”
Wolff also emphasizes the importance of being confident that your choice of cell-based assay is robust and reliable. “In the case of BsAbs, both target and effector cells are often necessary to emulate the mode-of-action,” he elaborates. “Thus, having stable cell lines is crucial for the robustness of the assay, and we typically optimize our cells to be ready-to-use. We also prioritize genetically engineered cell lines with reporter genes (e.g., luciferase) over traditional cytokine-releasing cell lines as they require less cumbersome detection methods (e.g., ELISA) which significantly reduces the response time.”
The future of cell-based assays in immuno-oncology
The use of BsAbs in the treatment of cancer and other diseases is an exciting new frontier, and cell-based assays currently play an important role in their development and look to be increasingly integral to the field of immuno-oncology in the future. “Cell-based assays are becoming more and more popular in immuno-oncology drug development due to their ability to mimic the complex interactions between tumor cells and the immune system,” Wolff explains. “The use of cell-based assays enables more accurate predictions of drug efficacy and safety compared to traditional preclinical models, ultimately resulting in the development of more effective and safer drugs.”
Wolff concludes, “overall, the continued development and implementation of cell-based assays in immuno-oncology drug discovery will play a crucial role in identifying new therapeutic targets, accelerating drug development, and improving patient outcomes.”