Consistency and quality in raw materials: Building blocks of a reliable in vitro diagnostic assay
Industry experts share insights on the elegant technology behind immunoassays and the critical raw materials that make them powerful
4 Mar 2021

Katie Spitere, product portfolio manager, Mary Saunders, product manager, and Terri Borree, portfolio manager, MilliporeSigma
While the world waits on COVID-19 vaccines to be distributed, diagnostic assays remain in high demand as testing continues in an effort to curtail the spread of the pandemic. Critical raw materials that go into building these assays are crucial to the specificity and sensitivity of these tests. Providing an exclusive insider’s perspective are product portfolio manager Katie Spitere, along with her colleagues, Terri Borree, portfolio manager, and Mary Saunders, product manager, who cover a breadth of critical raw material portfolios for diagnostic assays at MilliporeSigma.
Dual application in diagnosis and detection
Based on the principle of specificity behind an antigen-antibody reaction, immunoassays are a vital tool for detecting infectious diseases and are widely used within the design of diagnostic tests. In the case of the current pandemic, diagnostic tests are being used to assess if the patient has an active COVID-19 infection. While molecular tests such as RT-PCR assess this by detecting the virus’s RNA in a patient sample, immunoassays detect COVID-19 specific antigens. These antigen tests use antibodies in matched pairs to capture and detect antigens using common assay platforms such as lateral flow or ELISA. By detecting viral presence and producing results in a short turnaround, lateral flow assays can be used as point-of-care tests.
Another application of immunoassays is in detecting exposure to a virus, such as SARS-CoV-2, without directly testing for viral presence. Using lateral flow or ELISA platforms, serological tests achieve this by monitoring the immune response over time. In this case, the viral antigen is used to capture and identify the presence of antibodies in a bodily fluid such as blood. As Borree elaborates: “These tests can even be designed to differentiate an acute or recent infection from a past or prolonged infection. This is possible as the class of antibodies (known as IgM antibodies) that help the body fight off an infection the first time is different from the class (known as IgG antibodies) that potentially provides future immunity.”
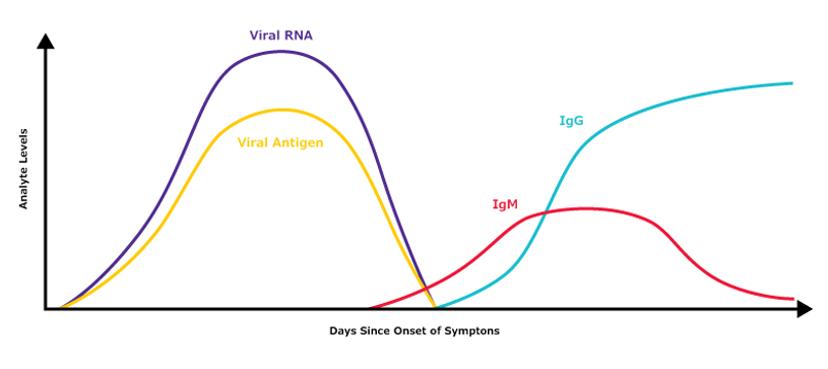
Estimate of general biomarker levels during the typical timecourse of COVID-19/SARS-CoV-2 infection. Please note that this is purely illustrative and should not be used as a primary reference.
A crucial factor that determines the sensitivity and specificity of these widely applied assays is the quality of raw materials involved. Membranes, antibodies, surfactants, detergents, protein blockers, buffers and detector particles form the core components of the test kits. Here, Spitere, Saunders and Boree share their thoughts on how critical raw materials are key to assay performance and development, and what they think the future holds.
Q: Why are critical raw materials so important for assay performance?
MS: Critical raw materials are the building blocks of a good assay. This is even more important with in vitro assays where the outcome directly impacts a patient. As a supplier of raw materials, we need to implement lot-to-lot consistency and proper controls to ensure that materials meet our standards as well as our customers’ standards.
KS: Having high-quality raw materials that reliably perform in a robust and reproducible manner ultimately ensures we meet the needs of the end patient in the best way possible.
Q: What are the key considerations for product development when it comes to in vitro assays?
MS: One of the key considerations is making sure the raw materials provide consistent results in the optimized test.
TB: It is important to have stability in terms of a reliable shelf life for raw materials. We also proactively engage in an open dialogue with our manufacturing partners to understand long-term demand. This would help ensure proper resource planning for both parties which in turn helps to mitigate the risk of supply.
KS: In addition to the above considerations, high-quality raw materials, as well as a robust supply chain, are essential.
Q: How has COVID-19 affected these considerations?
MS: I don’t believe that COVID-19 has changed our approach, but it has reaffirmed the importance of ensuring that the supply can meet demand. As some customers double or even triple their normal manufacturing capacity, they need to have confidence that their raw materials supplier can meet their requirements.
KS: Quality still remains key even with COVID-19. We are continually monitoring demand spikes and logistical challenges closely to ensure we mitigate risks and continue to meet our customers’ needs in the ever-changing COVID-19 landscape.
Q: What are the advantages of using critical raw materials from MilliporeSigma?
TB: At MilliporeSigma, we consider diagnostic assay manufacturers as partners rather than as customers. There is a strong focus on securing technical details and potential hurdles so we can identify the right raw material with the best formulation for a chosen assay and platform.
KS: We deliver high quality in terms of product, service and technical support.
Q: What areas of assay development can you support?
KS: Given our technical experience and intimate product knowledge, we can support our customers to ensure they choose appropriate raw materials to best suit their requirements.
MS: And selecting the right critical raw materials is always key as it can prevent having to re-tool an assay, which otherwise can be quite costly.
TB: Also, while our team doesn’t directly support it, if assay development resources are needed we recommend our partners to the contract manufacturing team in our larger diagnostics group. We promote a symbiotic ‘help me, help you’ attitude — customers trust us by sharing their assay details, and in return, we invest our time in directing them to the best product configuration.
Q: What are the bottlenecks or challenges facing lateral flow and IVD manufacturing capabilities?
MS: Having to manage the increase in demand, while continuing to work with safe working environments—exacerbated by pandemic restrictions—is a big challenge. Many manufacturers are approaching this issue by having extra shifts to de-densify the floor and instituting measures to abide by Centers for Disease Control and Prevention (CDC) recommendations. Many raw materials may also be short in supply due to the overall demand increase from the industry. Maintaining a secure supply chain is very important.
KS: Our manufacturing sites have been forced to adapt to COVID-19: in most cases, we have had to continue manufacturing with a reduced on-site presence. Given the far-reaching impact of this pandemic on our own vendors and the movement of material worldwide, we have closely monitored our supply chain. While it has been a challenge, we have managed successfully to date without any major disruptions in supply to our customers.
TB: Another critical challenge is mitigating our own supply risk by weighing customer demand against batch size risk, while also having contingency plans in place for manufacturing errors or batch failures.
Q: What do you see as the future landscape for rapid diagnostics and how is MilliporeSigma helping to enable this?
KS: COVID-19 has reaffirmed the critical need for high-quality, sensitive and reliable diagnostic tests. Continuing to partner with our customers to meet their needs as well as supplying robust raw materials is now more crucial than ever.
MS: We value collaboration. We understand and value the importance of partnering with customers that are making an impact in the diagnostics market through rapid testing.
TB: Rapid diagnostics will continue to evolve as platform technologies and materials move towards reduced sample sizes with shorter detection times. While this will require lesser reagent volumes, it will also come with a demand for greater sensitivity and specificity. To deliver this, MilliporeSigma scientists are using cutting-edge knowledge and skills in recombinant technologies to continuously optimize our portfolio for the needs of the future.
For more information on COVID-19 and the design, application, and manufacturing of lateral flow tests, see the related resources below.
Articles:
Application notes:
The Life Science business of Merck KGaA, Darmstadt, Germany, operates as MilliporeSigma in the U.S. and Canada
Request a free sample pack to see how Hi-Flow™ Plus membranes can enhance your tests.
