Drive cost-efficiencies in healthcare pathways with POCT
Highlighting the benefits that point-of-care testing can deliver for cost-efficient healthcare pathways
7 May 2024It is well understood that the use of diagnostic testing plays a vital part in guiding disease management and improving patient outcomes and wellbeing1. In recent studies, the successful implementation of point-of-care (POC) testing has shown substantial savings of between 8% and 25% across different settings2. The majority of POC testing-related savings have been shown downstream in the process of patient care3.
Accurate diagnostics, delivered at point of care can result in both clinical benefits for patients and economic benefits for the healthcare system4:
- Early detection: Identifying diseases in their early stages can help to initiate treatment sooner.
- Accurate diagnosis: Delivering lab-comparable results using devices at point of care,
- Effective monitoring: Serial measuring of biomarkers to track the progress of a disease or effectiveness of a treatment or therapy.
- Earlier intervention: Where some tests could be used to detect early risk factors and allow preventative measures to be taken.
How can POCT help?
Testing at point of care has proven to be a valuable counterpart to traditional laboratory testing2 and can be seen across the platform menu on the LumiraDx Instrument.
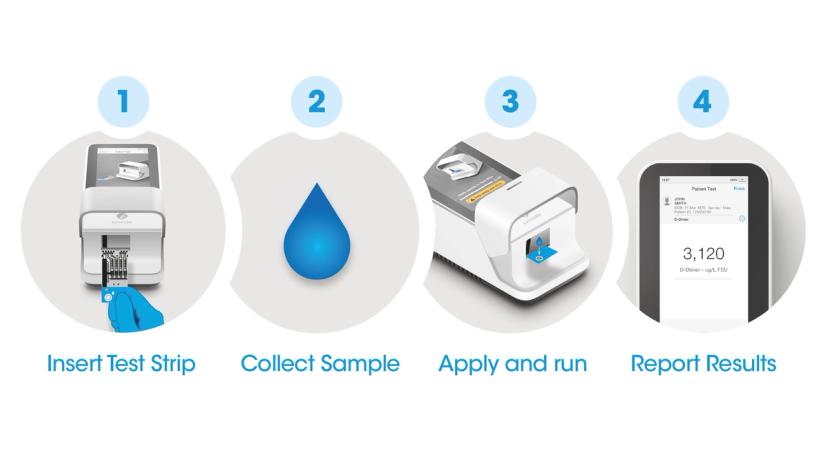
The LumiraDx Platform is the next generation of point of care systems which combines a small, portable instrument, and microfluidic test strip into a simple workflow.
This easy-to-use and highly portable instrument enables testing in almost any setting. It has a standardized workflow, which is consistent for all tests, so users can simply follow the on-screen video display for high-sensitivity results in minutes. With a broad test menu on one instrument, the LumiraDx Platform removes the need for multiple diagnostic instruments by integrating several technologies and sample types into a single instrument. Delivering lab-comparable performance at speed enables healthcare providers globally to strength community-based healthcare.
CRP in primary care and community-based settings
At present, rapid diagnostic tools are not widely used or effectively integrated into pathways, representing a significant area for change and innovation in practice that could progress the AMR (Antimicrobial Resistance) agenda5. C-reactive protein (CRP) testing can offer clinicians a rapid test to guide antibiotic prescribing decisions6 and using a POC CRP test in primary care has been shown to reduce antibiotic prescribing by 23–36% for RTIs7 and 22% for chronic obstructive pulmonary disease (COPD)8.
When considering where cost-savings can be made, health economic studies have shown that when compared to traditional clinical pathways, implementing POC CRP based strategies can save up to €7.94 per patient9.
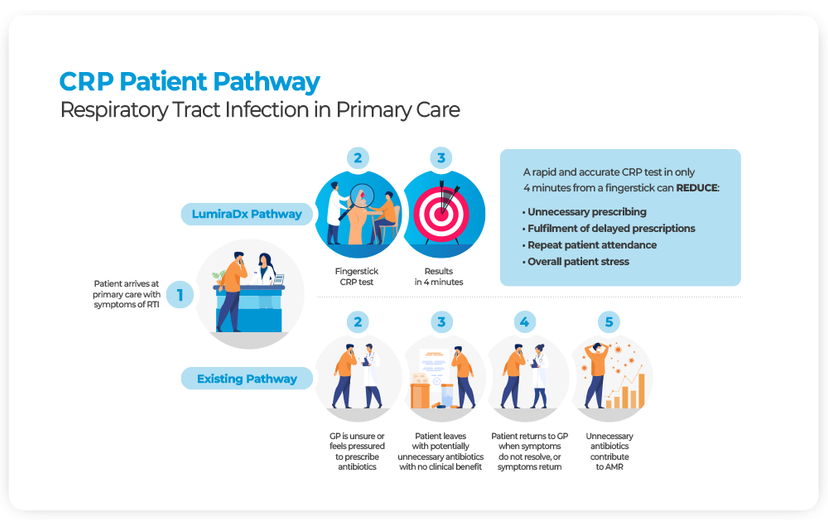
When considering the applications in the community, CRP testing can support real-time decision-making on the need for antibiotic treatment in frail, acutely unwell patients. This supports the avoidance of hospital admission, as rather than waiting for lab results several hours later POC CRP testing can inform immediate treatment change. In a case study by Frimley Health10, POC CRP improved the safety of care and helped avoid the need for escalation to hospital care in 50% of the patients assessed.
NT-proBNP in primary care and community-based settings
Heart failure (HF) is a leading cause of patient morbidity, disability, mortality and health inequality globally and diagnosis via the hospital admission-based pathway continues to dominate11. Compared to being diagnosed in the community, hospital admission-based diagnosis is associated with a significantly greater short-term risk of mortality and substantially increased long-term costs12.
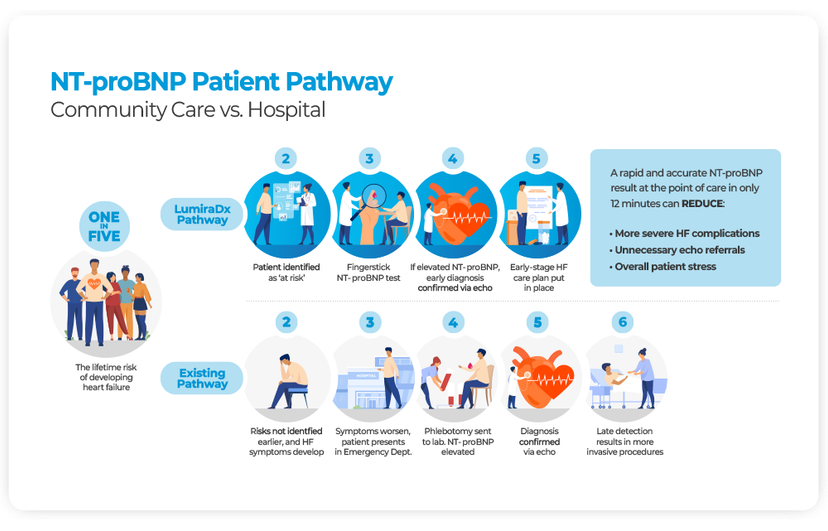
HF diagnosis via the hospital-based pathway, which typically occurs after patients have started experiencing severe symptoms, is estimated to incur an overall extra cost of £2,485 per patient12. Ruling out HF in primary care can reduce referrals to specialists and the requirement for more advanced diagnostic interventions by 25%13.
D-Dimer in primary care and community-based settings
Traditional pathways for the diagnosis of venous thromboembolism (VTE) involve multiple points of contact, and eventual diagnosis can take hours, or even days to achieve14. POC D-Dimer testing is used as an aid in the diagnosis of VTE and is widely accepted as the first step in the management of patients with suspected VTE15. A D-Dimer result can exclude patients from the VTE pathway in conjunction with a low-risk clinical pre-test probability score14.
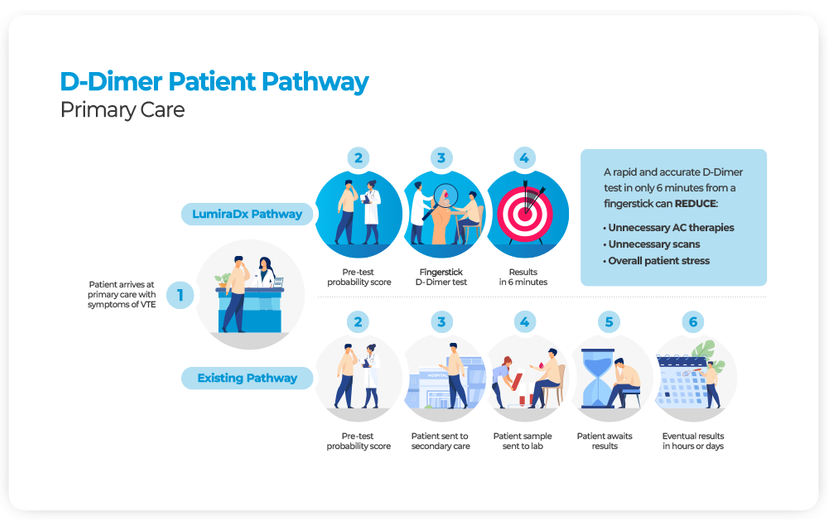
Using point of care testing for D-Dimer as part of a strategy to exclude DVT in primary care has been found to be cost effective compared to secondary care-based strategies16 where cost savings based on implementing POC D-Dimer based strategies compared to laboratory testing could be between €87 and €103 per patient3.
What lies ahead?
As we look to the future, with an ageing population who are often living with more than one health condition or illness, the costs of healthcare will continue rise unless changes and improvements are delivered across the systems. With point of care tests delivering accurate results, at the point of need, patients can be tested and treated earlier, faster and more efficiently than ever before.
The LumiraDx Platform allows for multiple analytes to be tested cost-efficiently and supports with reductions to hospital admissions, improving patient flow through pathways and managing health conditions.
References
1. Lingervelder D., Koffijberg H., Kusters R., IJzerman M.J. Health Economic Evidence of Point-of-Care Testing: A Systematic Review. Pharmacoecon Open. 2021 Jun;5(2):157–173.
2. Schilling U.M. The economic benefits of point-of-care testing. Hospital Healthcare Europe, special supplement. www.hospitalhealthcare.com
3. Heerink J.S., et al. Two point-of-care test-based approaches for the exclusion of deep vein thrombosis in general practice: a cost-effectiveness analysis. BMC Primary Care (2023) 24:42. https://doi.org/10.1186/s12875-023-01992-z
4. Jordan B., Mitchell C., Anderson A., Farkas N., Batrla R. The clinical and health economic value of clinical laboratory diagnostics. Ejifcc. 2015;26(1):47–62.
5. Testing Times Final Report on the role of rapid diagnostics in tackling antimicrobial resistance (AMR). 2022. In partnership with British In Vitro Diagnostic Association (BIVDA). Available at Testing Times lumiradx_testing-times_final-report_14.09.2022.pdf
6. A. GPs feel pressurised to prescribe unnecessary antibiotics, survey finds. BMJ. 2014 Aug 19;349:g5238. doi: 10.1136/bmj.g5238. PMID: 25143516. GPs feel pressurised to prescribe unnecessary antibiotics, survey finds | The BMJ
7. Cooke, J. et al. (2015) ‘Narrative review of primary care point-of-care testing (POCT) and antibacterial use in respiratory tract infection (RTI)’, BMJ Open Respiratory Research, 2(1). doi:10.1136/bmjresp-2015-000086.
8. Butler C.C., Gillespie D., White P., et al. C-Reactive Protein Testing to Guide Antibiotic Prescribing for COPD Exacerbations. N Engl J Med. 2019;381(2):111–120. doi:10.1056/NEJMoa1803185.
9. Hunter, R. Cost-Effectiveness of Point-of-Care C-Reactive Protein Tests for Respiratory Tract Infection in Primary Care in England. Advances in therapy. 2015. 32 (1); 69–85.
10. Frimley Park. Riddoch et al. Clinical Impact of POCT CRP testing in the Hospital@Home service. (2023) Frimley Health NHS Foundation Trust Poster
11. NHS England Guideline. Enhancing GP direct access to diagnostic tests for patients with suspected chronic obstructive pulmonary disease, asthma, or heart failure. Updated Jan 2024.
12. Bachtiger P., et.al. Survival and health economic outcomes in heart failure diagnosed at hospital admission versus community settings: a propensity-matched analysis. BMJ Health & Care Informatics. 2023.
13. Bayes-Genis, A. and Rosano, G. (2023), Unlocking the potential of natriuretic peptide testing in primary care: A roadmap for early heart failure diagnosis. Eur J Heart Fail, 25: 1181–1184.
14. Kearon C. Diagnosis of suspected venous thromboembolism. Hematology Am Soc Hematol Educ Program. 2016 Dec 2;2016(1):397–403. doi: 10.1182/asheducation-2016.1.397. PMID: 27913507; PMCID: PMC6142443.
15. Rodger M.A., Le Gal G., Wells P. et al. Clinical decision rules and D-Dimer in venous thromboembolism: current controversies and future research priorities. Throm Res 2014; 134,4: 763–68.
16. Price C.P., Fay M., Hopstaken R.M. Point-of-Care Testing for D-Dimer in the Diagnosis of Venous Thromboembolism in Primary Care: A Narrative Review. Cardiol Ther. 2021 Jun;10(1):27–40. doi: 10.1007/s40119-020-00206-2. Epub 2020 Dec 2. PMID: 33263839; PMCID: PMC8126530.

