Efficient scale-up is the key to efficient commercial formulation of lipid nanoparticles
In this guest editorial, Denis Smit from Micropore Technologies outlines the challenges faced in lipid nanoparticle development and manufacturing
7 Jun 2022
This guest editorial by Denis Smit from Micropore Technologies outlines the challenges in being able to achieve the same product performance and critical quality attributes from discovery through preclinical and clinical testing, and finally on to the full-scale manufacturing of lipid nanoparticles (LNPs).
‘What might be acceptable in a pandemic is not likely to remain so beyond it’

At a recent lipid nanoparticle (LNP) formulation and process development summit held in Boston, a delegate observed that “utilizing mRNA-loaded LNPs is probably the most complicated drug product that humans have ever tried to manufacture.” While the COVID-19 pandemic enabled the remarkable proving of mRNA-LNP technology, given its complexity and the need for rapid deployment, it was to be expected that opportunities for improving this technology pathway would remain. A key driver within this modality is the Pandora’s box of opportunities that this biopharmaceutical technique has opened – from a multitude of infectious diseases to somatic and germ line genetic diseases. This dictates the need for second-generation technologies, both in formulation and process development, to address remaining challenges and the needs for improvement. Simply put, what might be acceptable in a pandemic is not likely to remain so beyond it.
‘Process steps that one might hope would be inconsequential almost never are’
When it comes to manufacturing biopharmaceuticals like LNPs the importance of a holistic approach is now clear. Individual unit operations can and do affect critical quality attributes of the final product – indeed, as another delegate at the afore mentioned conference observed, “process steps that one might hope would be inconsequential almost never are.” Operability, control (including process analytical technology (PAT) feedback loops), and maintenance aspects of the whole process are required to minimize variability of the final product. Unit operations that are difficult to control, operate, or that have significant maintenance requirements, make design of the complete process challenging.
So, when starting to work on the first discovery stage experiment, the ability to envision a scalability pathway – confident that success will translate to full-scale GMP manufacturing – is important. Results from each stage in the preclinical and clinical phases must accurately translate all the way through to final GMP manufacturing. Challenges here include assays whose results change from in vitro to in vivo and, frustratingly, between animal species, and changes in particle size and size distribution which can affect not only yield, but tissue targeting and transfection efficiency.
‘No detectable degradation to mRNA’
Micropore Technologies first commercialized its continuous manufacturing-scale crossflow micro mixing technology in 2019. Born out of over a decade’s experience in aseptic processing of microspheres, in late 2021, it introduced a single device specifically engineered for early-stage discovery through to clinical trials – the AXF-mini. In short, we started with the scaled-up version of our equipment and then grappled with the challenge of how to scale it down.
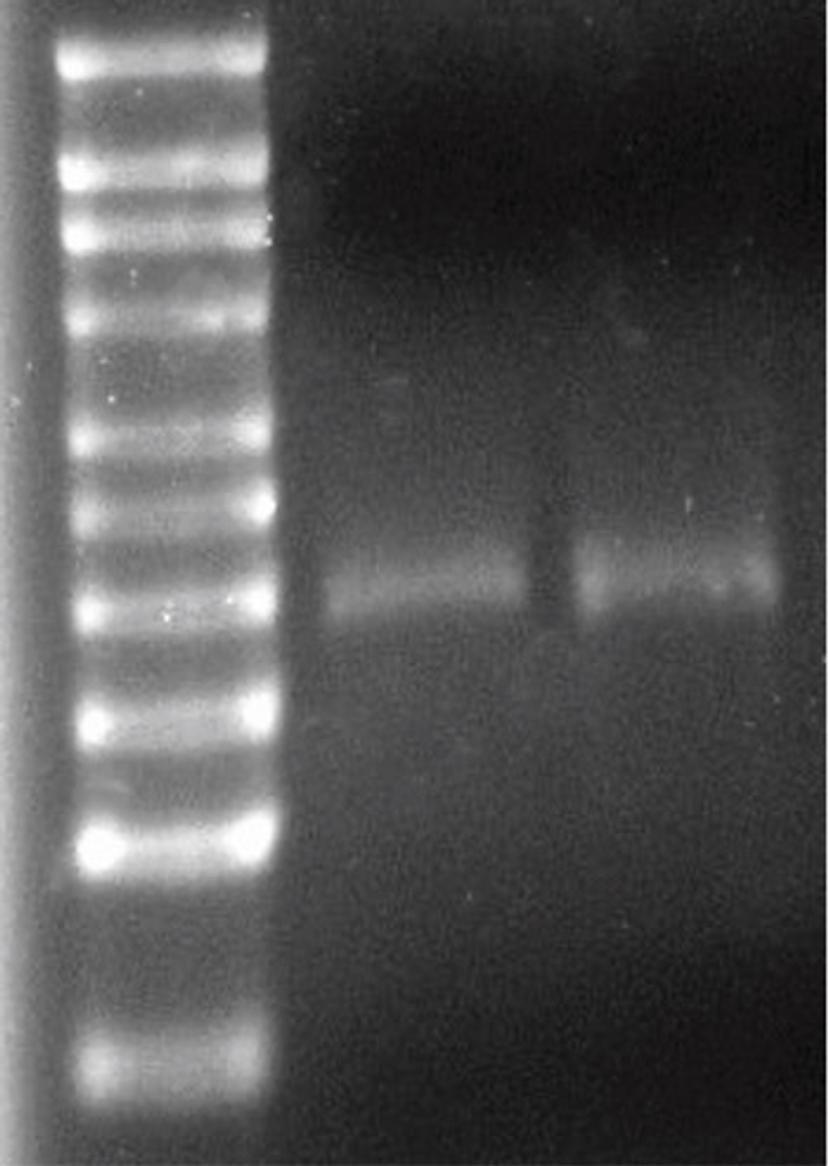
Operating at low pressure (requiring typically 85% lower energy than alternative technologies), independent research at the University of Strathclyde in Scotland lead by Dr Yvonne Perrie has demonstrated that Micropore’s membrane-based crossflow devices deliver no detectable degradation to mRNA, which is widely acknowledged to be very sensitive to energy inputs and particularly so for the larger oligonucleotides like saRNA. This is an exciting result for vaccine manufacturers because it demonstrates that crossflow membrane mixing could open the door to the cost-efficient mass production of a whole new generation of LNP-based therapeutics.
Consistent particle sizes, as small as 35 nm, from 0.5 mL samples to liters-per-hour production runs
While most LNPs target 100 nm, Micropore’s ongoing research into particle formation using its advanced crossflow technology has demonstrated that different formulations can deliver particles as small as 35 nm and at volumes from approx. 0.5 mL samples to many liters-an-hour production runs with the same uniform consistency throughout. For biopharmaceutical developers and manufacturers, this technology will enable products to be manufactured within a targeted particle size distribution from which a robust manufacturing scale skid can be designed and built using a scale-up approach with the same conditions (low pressure and low shear) and the same physical mechanisms (driven by a constant and low Reynolds number), while maintaining the same geometry all the way through the process drug of development to manufacturing.
The development of LNP technology to the point where it is a recognized method of delivering many different oligonucleotide payloads has spurred Micropore Technologies to develop further products that speed up the LNP formulation process and a new AXF-DoE device is expected to be released in the second half of 2022. This will meet the brief of being able to generate 300–500 microliter samples while being scalable to 20 L/h and beyond.

