Elephants in the Room
by Mark Clymer, Datacolor Scientific
5 Feb 2016
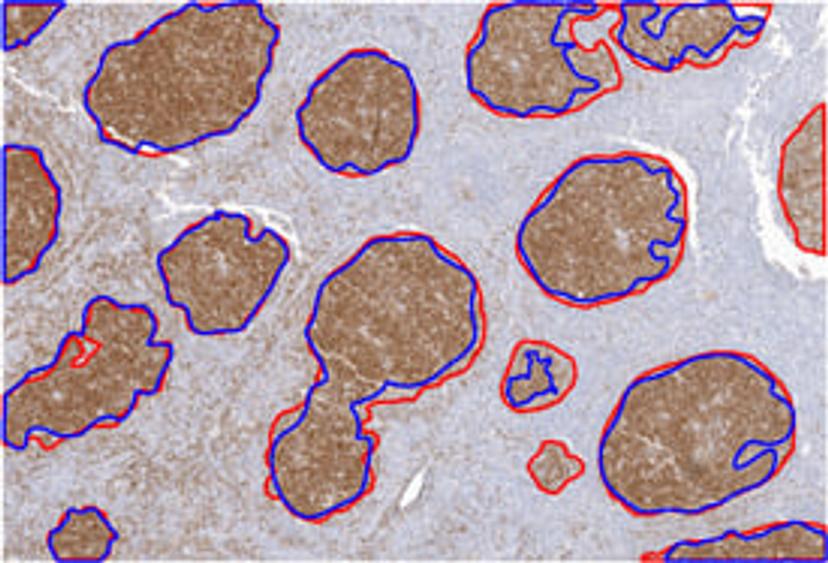
Image courtesy of Ohio SuperComputer Center via www.sciencedaily.com.
In mid-January the Digital Pathology Association (“DPA”) announced that the ‘de novo’ application process may be acceptable by the FDA for clearance of whole slide imaging devices (“WSI” devices) for primary diagnostic uses. Why is this significant? The de novo process lowers the primary hurdle for marketing approval from that of a class III device to essentially a class II device.
But WSI device manufacturers aren’t out of the woods yet. There are other proverbial “elephants in the room” with which they’ll have to contend.
Batter up
With medical devices, the manufacturer who is first-to-market generally bears the cost burden to conduct the clinical trials. But somebody has to submit first. Will WSI device manufacturers actually cooperate to share the high cost of the first approval, speeding approval of all WSI device applications?
Where have all the pathologists gone?
OK, so maybe it won’t be that dramatic, but it’s just a matter of time before computer-aided diagnosis (“CADx”) augments the digital pathology practice. For the time being, human eyes would continue to evaluate and interpret the digital renderings of patient specimens on computer monitors. Human perception and trained intuition will not be replaced by computer algorithms anytime soon; however, data-driven diagnosis and healthcare will prove to improve the quality of care for patients. As long as image quality is consistent and guaranteed, CADx would have the advantage of eliminating the displayed image from the equation, basing the analysis on the actual image data.
Of all of our inventions for mass communication, pictures still speak the most universally understood language
Walt Disney
One of the biggest challenges with digital pathology is observation of the digital image. Computer monitors vary greatly in their ability to render images accurately, and that’s assuming that the image is indeed an accurate representation of the actual specimen (accurately captured, digitized, saved and communicated). All monitors suffer from a deteriorating ability to render colors, and all benefit from regular and periodic calibration.
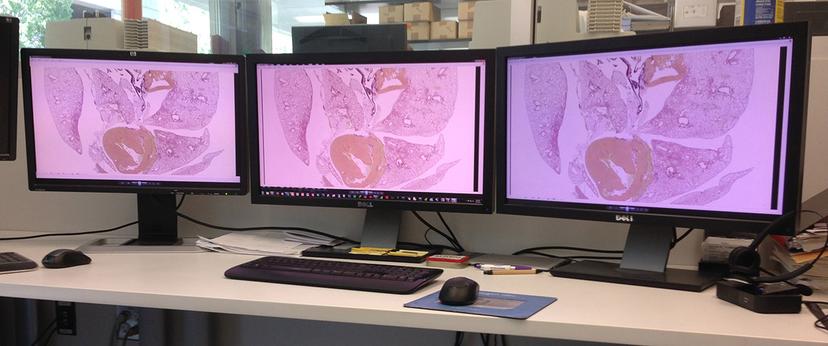
From another perspective, a well-calibrated monitor may actually reveal deficiencies in image quality (i.e. detail, color balance, saturation, contrast, etc.), or in specimen preparation. To achieve this “universally understood language” for pathology, pathologists will experience a period of acclimation while they adjust to the new digital viewing environment.
Quality controls
The FDA expects to address quality through a combination of manufacturer-established quality systems, including change control, and technical performance assessments. It’s been nearly a year since the FDA issued its draft guidance for technical performance assessment of WSI devices, and industry representatives, practitioners and the FDA have been working to elaborate on the planned assessments. The final guidance will need to be in place in advance of any marketing approval for a de novo application.
Thanks to Mark Clymer, Datacolor Scientific
