Exploring the power of super-resolution microscopy: Unveiling the unseen
Discover how single-molecule localization microscopy can image subcellular structures deep within samples to reveal novel biological insights
31 Jul 2023
Microscopic imaging has revolutionized scientific research by allowing scientists to delve into the intricate details of biological specimens that are otherwise invisible to the naked eye. With the advancement of super-resolution microscopy techniques, such as single-molecule localization microscopy (SMLM), researchers are able to generate exceptionally high-resolution images that provide invaluable insights across various disciplines that are not achievable with other microscopy methods. In this SelectScience® interview, we speak with Winfried Wiegraebe, Ph.D., Product Manager of Super-Resolution Microscopy at Bruker, who explains the benefits of this groundbreaking technology, how it works, and its applications in different research fields. Wiegraebe also shares key considerations for selecting an SMLM microscope and highlights the cutting-edge capabilities of SMLM microscopes from Bruker.
Light microscopes have been the workhorse in laboratories for decades. They work by using visible light and a system of lenses to generate magnified images of biological specimens. However, light microscopes are constrained by the diffraction limit, which restricts their resolution to a few hundred nanometers. Consequently, intricate details of intracellular structures and activities remain unresolved, posing challenges across many research fields. Super-resolution microscopy techniques – including SMLM – have emerged as a solution to overcome these limitations. By surpassing the diffraction limit, SMLM allows scientists to visualize individual molecules with resolutions below 20 nm, unveiling cellular dynamics and nanostructures previously inaccessible.
“Having the ability to visualize minute specimens in super high resolution has helped scientists answer many fundamental questions that contribute to important scientific advances,” Wiegraebe explains. “Sometimes the answer lies right there, but it’s not always possible to see it because many subcellular structures and other important molecules are far too small to resolve with standard microscopy.”
Generating high-resolution images with single-molecule localization microscopy
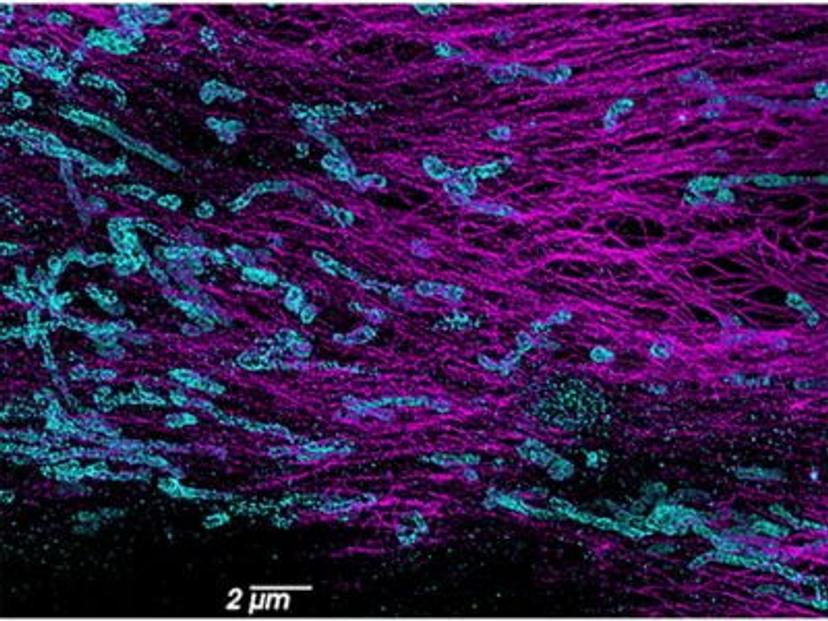
In conventional light microscopy, when imaging a sample densely labeled with fluorescent molecules, the resulting image appears as a blurred fluorescence signal without a clear resolution of the underlying structure. This limitation is due to the fact that all molecules within a certain proximity are simultaneously imaged, resulting in overlapping signals. SMLM overcomes this diffraction limit by collecting data from individual molecules separately. It creates a blinking effect by stochastically exciting each molecule at different time points, which means that only a small subset of molecules is "turned on" or emitting fluorescence at any given time. This generates sparsely distributed point spread functions (PSFs) that can be individually localized. The precise localization of each molecule is determined through fitting algorithms, which extract spatial information below the diffraction limit, providing high-resolution positional data for each molecule. Using this information, a super-resolution image is constructed that reveals the fine details of a sample.
“SMLM offers the highest resolution and quantification capabilities among other super-resolution techniques, such as structured illumination and stimulated emission depletion (STED) microscopy,” adds Wiegraebe. “Although electron microscopy (EM) can also provide extremely high resolutions and is able to capture the context and fine structures of samples, unlike SMLM it lacks the ability to precisely identify labeled molecules.”
There are two main strategies for controlling the fluorescence of fluorophores: using fluorescent proteins that can be photoactivatable or photoconvertible and using organic fluorescent dyes that spontaneously start blinking under the correct conditions. Examples of techniques using fluorescent proteins include photoactivated localization microscopy (PALM), and those that use fluorescent dyes include stochastic optical reconstruction microscopy (STORM) and direct-STORM (dSTORM), and points accumulation for imaging in nanoscale topography (PAINT).
Expanding our understanding in multiple scientific fields
Super-resolution imaging has found numerous applications in different research disciplines including neuroscience, cell biology, genomics, and virology research.
“SMLM is ideally suited for cell biology research because it allows researchers to observe subcellular structures that cannot be imaged with other microscopy methods. This enhances our understanding of the inner workings of cells, which is essential for disease modeling and the development of treatments,” explains Wiegraebe.

Delving even deeper, SMLM can be used to study DNA, RNA, and proteins to provide key insights into cellular pathology. This includes the study of macromolecular complexes; super-structures; chromatin structure; chromosomal substructures; the organization of genomes; and the structure and regulation of genetic information within a single nucleus.
“What’s more, SMLM supports spatial biology research on viruses and extracellular vesicles (EVs) by shedding light on their interactions, intracellular movement, and structures, which is otherwise unobservable because they are below the resolution limit of other microscopes,” adds Wiegraebe.
SMLM is showing particular promise in the field of neuroscience, as it enables detailed studies of synapses. This insight can help improve scientists’ understanding of neurological and psychiatric disorders to inform the development of innovative new treatments. In addition, the application of SMLM in live biological imaging – where structures and biological processes can be observed within cells in real-time – is crucial for answering several modern research questions.
The most advanced super-resolution microscope
When choosing a super-resolution microscope, it’s important to ensure the optics are optimized for maximum resolution and it has a high level of stability – if there is even a small amount of drifting or shaking then the resolution will be affected. Many standard microscopes can be modified to perform high-resolution imaging; however, they are not specifically designed for this function and do not perform as well as a dedicated super-resolution microscope.
SMLM microscopes from Bruker, such as the Vutara VXL, are designed to offer superior resolution performance and ensure stability by using a single objective and controlling temperature gradients. Thanks to Bruker’s robust biplane technology, the Vutara VXL can achieve the deepest 3D imaging with every acquisition and can perform SMLM on more sample types than any other commercial single-molecule localization microscope available. The integration of the PlexFlo Multiplexing Platform expands the system's multiplex capabilities and enables sequential labeling for more advanced experimental designs.
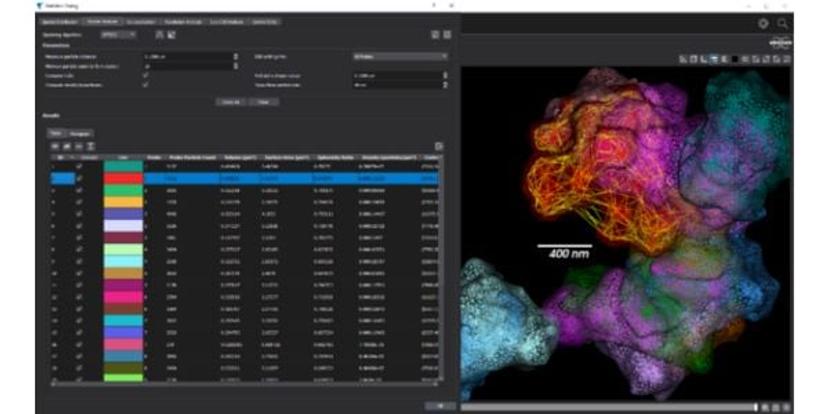
The Vutara SRX software empowers researchers to extract visual and quantitative information from biological samples. By localizing individual molecules, the Vutara VXL not only generates stunning 3D images but also provides in-depth quantitative analysis tools, thus enhancing the ability to extract meaningful insights from their data.
“User-friendliness is important for core facility operation,” states Wiegraebe. “Bruker's workflow-defined software simplifies experimentation and enables seamless control of the SMLM microscope. Our application scientists also offer extensive support to customers by helping train new laboratory staff on how to use the SMLM microscopes. Furthermore, with a brief half-hour demo, most users are equipped with the skills to independently operate the system.”
Looking to the future
The future of super-resolution microscopy holds immense potential for further breakthroughs in multiple life science fields. While some research benefits from one method of microscopy alone, correlative microscopy combining different methods is becoming more beneficial as research advances. EM used alongside SMLM allows for both targeted labeling and ultra-high-resolution imaging. This approach enables researchers to collect rich and in-depth data beyond what any single technique can deliver.
“As microscopy continues to advance and we are able to examine biological samples at even higher resolutions, we gain a deeper understanding of complex biological processes,” concludes Wiegraebe. “This knowledge is essential for the development of new drugs, treatment strategies, and interventions that can significantly improve patient health outcomes.”
