High-sensitivity troponin at the point of care
Professor of Emergency Medicine at the University of Manchester highlights the promise of point-of-care high-sensitivity troponin assays in the emergency department
27 Jun 2024
Dr. Rick Body, Professor of Emergency Medicine at the University of Manchester, and Consultant in Emergency Medicine at the Manchester University NHS Foundation Trust
Symptoms of chest pain, indicative of possible acute myocardial infarction (AMI), are one of the most common causes of emergency department (ED) presentations worldwide. In England and Wales alone, approximately 1.3 million patients present to hospitals with chest pain every year. However, less than 15% of patients investigated are found to have myocardial infarction1. Suspected myocardial infarction thus imposes a significant healthcare burden, prompting efforts to develop diagnostic strategies that can accurately rule out AMI at an earlier stage. By implementing these strategic approaches, healthcare providers can proactively sidestep potentially unwarranted hospital admissions, alleviate the burden on EDs, and ultimately enhance both patient experiences and outcomes.
In the last decade, significant progress towards this goal has been made through the introduction of high-sensitivity cardiac troponin (hs-cTn) assays. Compared to older, standard troponin tests, these assays can detect much lower levels of troponin and smaller changes to a patient’s troponin levels, thus enabling earlier AMI diagnosis and improved risk stratification of patients with suspected acute coronary syndrome (ACS). Consequently, laboratory-based hs-cTn assays have been widely implemented in hospitals worldwide. More recently, new devices able to sensitively measure cardiac troponin levels within minutes at the patient’s side have become available, setting the scene for the next major evolution in troponin-based diagnostics: high-sensitivity cardiac troponin at the point of care.
In this article, we speak with Dr. Rick Body, Professor of Emergency Medicine at the University of Manchester, and a Consultant in Emergency Medicine at Manchester University NHS Foundation Trust, to learn more about the impact point-of-care (POC) high-sensitivity troponin assays could have on clinical decision-making, patient outcomes, and ED overcrowding. Dr. Body also shares his ongoing research evaluating the diagnostic accuracy of the Siemens Healthineers Atellica® VTLi high-sensitivity cardiac troponin assay and reveals why close collaboration between clinical labs and acute care physicians will be evermore important as POC testing continues to gain prominence.
Unveiling the crucial role of cardiac troponin testing
Cardiac troponin, a protein released into the bloodstream upon cardiac muscle death, is the biomarker recommended by guidelines to support the diagnosis of acute myocardial infarction. “There are other markers that have shown promise, such as copeptin and heart-type fatty acid binding protein,” notes Dr. Body. “However, cardiac troponin is such a good biomarker, and the available assays are so sensitive and precise that we don’t currently need anything else.”
“When making a clinical decision, we also take into account other important information, including the nature of a patient’s symptoms, their previous medical history, their risk factors for heart disease, physical examination findings, vital signs, and ECG findings,” he adds. “We use T-MACS, a computerized decision aid, which was developed through machine learning to allow us to use all this information in an evidence-based manner, calculating the probability of a heart attack for each patient and promoting a personalized medicine approach.”
By integrating high-sensitivity troponin assays and ECG findings with risk assessment aids such as T-MACS, the HEART score, and the TIMI score, clinical decisions for suspected AMI can be made much faster than previously achievable with standard troponin tests2–5. For this reason, high-sensitivity troponin testing has become increasingly adopted worldwide, and in the UK, several laboratory-based hs-cTn assays are recommended by the National Institute for Health and Care Excellence (NICE) as options for the early rule out of non-ST-segment elevation myocardial infarction (NSTEMI). “We now have superb evidence-based care pathways to allow us to rapidly differentiate patients who have a serious condition and need to receive care in a hospital from those who do not,” says Dr. Body. “The biggest challenge now is being able to use those pathways effectively amid the crowding and long waiting times that we currently see.”
If we can make rapid decisions, it means that patients will get to where they need to be – whether that be a hospital ward or their own home – faster.
Dr. Rick Body University of Manchester
Expediting critical care: Reducing vein-to-brain time with point-of-care devices
While hs-cTn assays have drastically shortened the timeframe required to safely rule out AMI, one roadblock for rapid decision-making has been prolonged turnaround times of results from central laboratories, primarily due to the transport, handling, and processing of blood samples6. This, compounded with the mounting pressure facing EDs, can considerably increase the time taken for results to be used in clinical decision-making, known as the vein-to-brain time. “It’s important that, where possible, clinicians have all the information they need to decide for a patient when they first see that patient,” explains Dr. Body. “If you need to end the consultation to order more tests and then return to the patients once the results are back then you’ll have inevitable delays. Doctors also get busy and distracted by other patients, which means patients must wait again to get their results even after they’ve become available.”
This is where POC testing offers enormous potential. “A rapid near-patient test that could provide results before the end of a consultation with a clinician would allow us to make rapid decisions, reducing waiting times for patients and helping to ease the growing problems we have with ED crowding,” Dr. Body continues. “If we can make rapid decisions, it means that patients will get to where they need to be – whether that be a hospital ward or their own home – faster. Moreover, by having the information to hand when we first see a patient, we can help to reduce unnecessary admissions to hospital wards, saving valuable beds and avoiding unwarranted stress among patients.”
POC hs-cTn testing also presents opportunities to deliver care in new ways. “We currently rely on central laboratory testing to measure cardiac troponin, meaning that patients need to come to larger hospitals with central laboratories,” explains Dr. Body. “With POC hs-cTn, we could run the same tests without a central laboratory, and that expands the possibilities for how we deliver care.” One potential application he notes is in prehospital settings, where POC hs-cTn testing could enable the evaluation of suspected AMI in the community and redirection of high-risk patients to cardiac centers and low-risk patients to district general hospitals. “We could do the diagnostic work-up in an ambulance or ambulatory care facility, for example, and this could have a huge impact on ED crowding,” he adds.
A potential game-changer
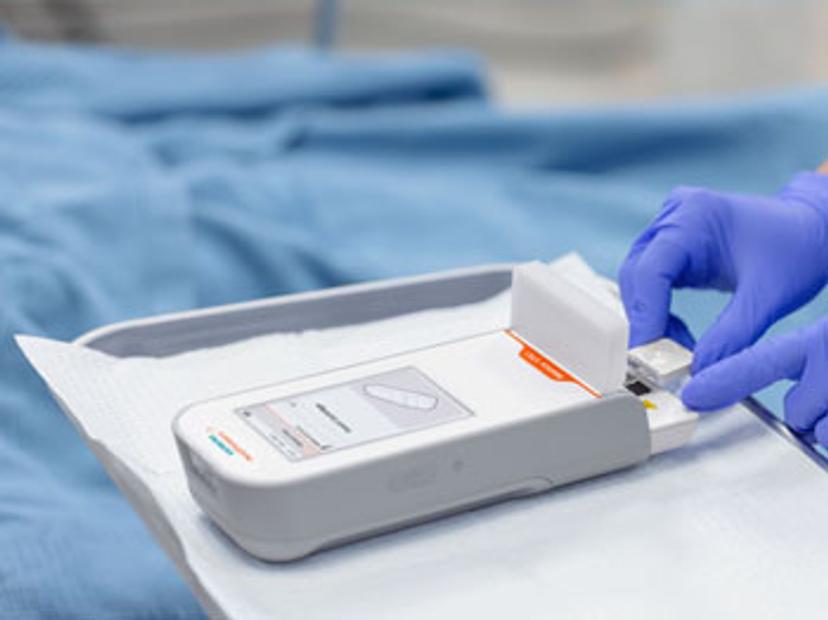
Simple to use and easily integrated into existing systems, the Atellica VTLi Patient-side Immunoassay Analyzer, puts accurate, clinically actionable hs-cTnl results directly in the hands of those who need it most
Point-of-care cardiac troponin testing is not a new idea. In the past few years, several devices have been developed to deliver the analytical benefits of high-sensitivity troponin assays in a shorter timeframe and a format suitable for non-laboratory personnel. However, until very recently, these POC assays have fallen short of meeting the established analytical performance criteria of laboratory-based hs-cTn tests.
Now, a novel point-of-care high-sensitivity troponin assay developed by Siemens Healthineers, called Atellica® VTLi Patient-side Immunoassay Analyzer, has become one of the first CE-marked POC hs-cTnI assays shown to deliver results comparable to laboratory performance. Relying on a simple venous or fingerstick sample of whole blood with result turn-around times in as short as eight minutes, the Atellica VTLi System is poised to be a game-changer, with the potential to revolutionize care delivery for millions of patients worldwide. The system also offers direct connectivity to data management and the LIS/HIS, so that laboratories can easily maintain centralized control of their decentralized testing.
The Atellica VTLi has already shown early promise in its first published evaluations6–7, however, multiple prospective clinical studies are required to fully validate its diagnostic accuracy in real-world settings. To this end, Dr. Body is conducting a Second Bedside Evaluation of Sensitive Troponin (BEST-2) study, recruiting patients in the ED who have presented with suspected cardiac chest pain. “We’re running the test on arrival and one hour later and collecting all the data we need to see if the test works well alongside existing decision aids like T-MACS or the HEART score,” he explains. “BEST-2 is taking place at seven hospitals across England and Scotland, and we hope to finish participant recruitment by the end of summer 2023.”
“So far, my experience has been incredibly positive,” Dr. Body enthuses. “It’s fantastic to see troponin results popping up within minutes of seeing the patient, and we’ve been able to detect heart attacks in several patients who were facing long waits in crowding waiting areas. Already, that’s meant that we could care for them in a more appropriate high-dependency environment while we’re waiting for the routine lab results.”
Navigating the path forward in POC
While early signs are promising, the journey toward implementing POC hs-cTn assays is not without its challenges. POC hs-cTn assays currently entail higher costs compared to traditional laboratory-based tests, and successful integration will require upfront investment in equipment, training, IT infrastructure, and quality control measures. Crucially, to realize the benefits of POC hs-cTn assays, care pathways must be re-designed to effectively leverage the rapid turnaround time to results. This may involve adjusting clinical consultations to occur at or closer to the time of testing, enabling timely decision-making and interventions based on the obtained results.
As POC testing gains prominence in clinical settings, Dr. Body also emphasizes the increasing importance of collaboration between lab personnel and acute care physicians. “We already use point-of-care testing for glucose, blood gases, and, to some extent, pregnancy testing,” he says. “However, as the menu of tests and the number of devices increases, we’re going to need to work ever closer to ensure that we have all the appropriate governance, quality controls, and pre-analytics covered.”
“This will be particularly challenging when we start to use point of care testing in new environments such as the ambulance service, where there may not be a dedicated point of care testing team,” he adds. “But these are incredibly exciting challenges to be facing, given the possibilities to improve patient care,” he concludes.
References:
1. Goodacre, S., et al. The health care burden of acute chest pain. Heart 91.2 (2005).
2. Than, M., et al. A 2-h diagnostic protocol to assess patients with chest pain symptoms in the Asia-Pacific region (ASPECT): a prospective observational validation study. The Lancet 377.9771 (2011).
3. Frisoli, T.M., et al. Henry Ford HEART score randomized trial: rapid discharge of patients evaluated for possible myocardial infarction. Circulation: Cardiovascular Quality and Outcomes 10.10 (2017).
4. Greenslade, J.H., et al. Widespread introduction of a high-sensitivity troponin assay: assessing the impact on patients and health services. Journal of Clinical Medicine 9.6 (2020).
5. Thygesen, K., et al. Universal definition of myocardial infarction. circulation 116.22 (2007).
6. Xiong-Hang, K., et al. Analytical performance comparing siemens whole blood point of care Atellica VTLi to the central laboratory plasma Atellica IM high-sensitivity cardiac troponin I assays. Clinical Biochemistry 114:79–85 (2023).
7. Apple, F.S., et al. Single high-sensitivity point-of-care whole-blood cardiac troponin I measurement to rule out acute myocardial infarction at low risk. Circulation 146(25):1918–1929 (2022).
Atellica VTLi Patient Side Immunoassay Analyzer is not available for sales in the U.S. Please contact your local Siemens Healthineers representative for availability.

