How Acid Purification Can Benefit Your Laboratory
20 Mar 2015

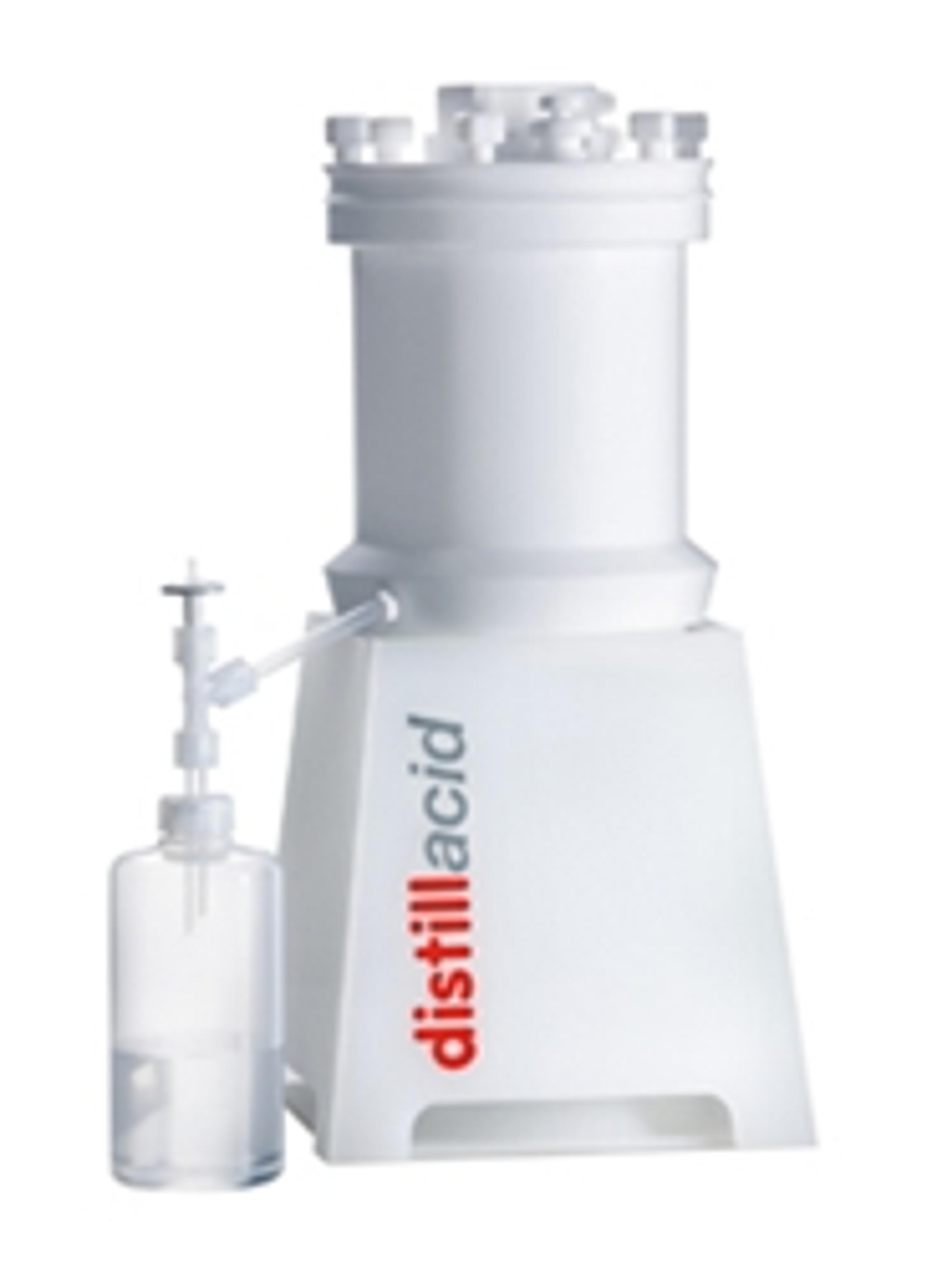
Subboiling apparatus BSB-939-IR for acid purification.
Reducing the cost of laboratory chemicals, while maintaining quality, is a major consideration for many laboratories. Ultra pure acids are used in many research applications; however, purchasing this grade of acid can be expensive. Using an acid purification system can significantly reduce the cost of purchasing ultra pure acids. In this exclusive article, you will discover the benefits of using an acid purification system for your laboratory.
1. Construction material
It is important that the risk of corrosion is minimized to ensure the purity of the acid produced is not compromised. This means that the materials the acid comes into contact with should be corrosion resistant, such as plastics. Fluorinated polymers such as PTFE and ultrapure PFA offer excellent chemical stability.
2. Sub-boiling principle
The sub-boiling principle can be used to produce high-purity acid for ultra trace analysis. This technique uses mild heating, which reduces the formation of droplets and the subsequent transportation of impurities. The low temperature also results in low boiling substances, such as salts, remaining as residue that does not contaminate the distillate.
The mild heating prevents the production of droplets and thus the conveyance of impurities. All low boiling substances, especially salts, remain in the residue, so you can benefit from the high degree of purification.
3. Cost effectiveness
The purification of low purity grade acids, which are more economic to purchase, can save up to 90% of the cost of purchasing analytically pure acids. This can effectively pay for an acid purification system within one year.
4. Application
All low boiling acids are suitable for distillation using the sub-boiling principle. It is also possible to produce high purity water very easily. High purity acids (nitric acid, hydrochloric acid and hydrofluoric acid) have a wide range of applications in many laboratories.
5. Distillation volume
Acid purification over a 24-hour period can produce a good volume of ultrapure acid. The following distillation quantities can be obtained: 1.2 L of nitric acid, 1.1 L hydrochloric acid, 1.0 L hydrofluoric acid and 1.8 L of water in 24 hours.