How Advanced Confocal Imaging Revealed a Key Role for the Cytoskeleton in Pathology of Heart Disease
Dr Ben Prosser discusses his mechanobiology research, recently published in Science
12 Jul 2016
Dr Ben Prosser (third from the right), an Assistant Professor in the Department of Physiology at the University of Pennsylvania Perelman School of Medicine, spoke to SelectScience® about his research into the role of the cytoskeleton in heart disease. The lab is focused on mechanobiology of the heart, more specifically how the cytoskeleton of cardiac cells influences the mechanics of the heart and the ability of cells to sense and respond to changing mechanical forces.
Using advanced imaging techniques Dr Prosser’s team recently published findings that the cytoskeleton of heart muscle cells (cardiomyocytes) has considerable effects on the mechanics of the heart and its contractility. The group demonstrated that the microtubules in these cardiomyocytes, classically believed to be stiff, rod-like structures, undergo dynamic, large, geometric changes as heart cells contract, and that they actually more closely resemble spring-like elements. The team also showed that specific modifications to the microtubules could affect the beating of cardiac cells and their responses to changing mechanical forces. These modifications are able to influence the mechanics of the heart and indicate that microtubule function has an important role in heart disease pathology and as a potential therapeutic target.
Advanced live cell imaging of the microtubule network
Visualizing the microtubule network of cardiomyocytes using fluorescent probes and confocal microscopy is relatively simple, however, capturing the behavior of the microtubule network during a contraction is not. High spatial and temporal resolution is required to capture these dynamic events, which occur in <100 ms and in microtubules, which are approximately 25 nm in diameter. As Dr Prosser explains, “ZEISS LSM 880 with Airyscan was pivotal for us.” The superresolution of LSM 880 with Airyscan allowed a larger field of view to be imaged, with high signal-to-noise. The addition of the Fast Acquisition Mode, maintains that high signal-to-noise for rapid imaging which enables microtubule network behavior to be monitored during a heartbeat.
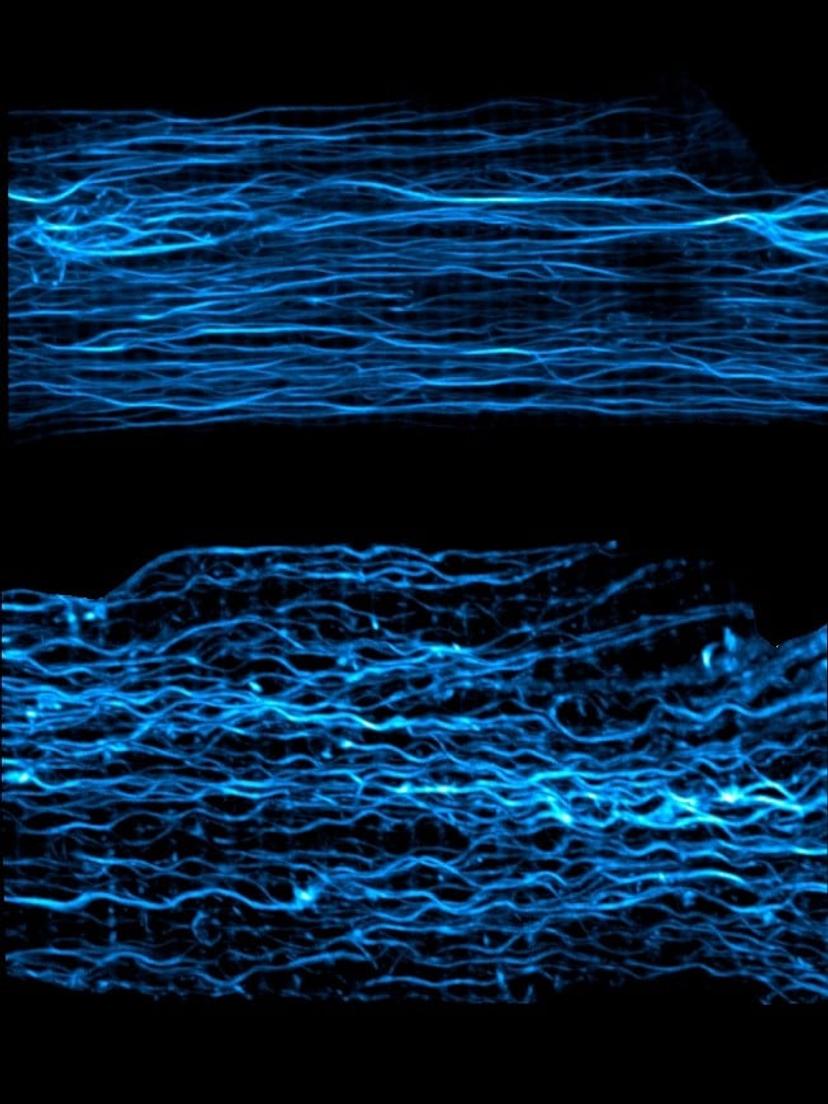
Fast Acquisition Mode LSM 880 with Airyscan image of cardiac cell microtubules at rest (top) and when the cell is compressed, as during contraction (bottom). Image courtesy of Dr Ben Prosser
The team are interested in dynamic changes in the structure of microtubules and use the Fast Acquisition Mode for the LSM 880 with Airyscan to track growth and shrinkage of microtubules in constantly beating heart cells, to see how this affects the microtubule network. In heart disease, where there is elevated blood pressure and increased cardiomyocyte stretching, differences in the cytoskeletal organization and density are evident. Using the fast mode, Dr Prosser’s team are now able to study this at the single cell level and show how the effects on the cytoskeletal network have a potent effect on the heartbeat.
Microtubule network changes as heart disease biomarkers
The team examined cardiac tissue samples from patients with different origins and severities of heart disease and screened for changes in their microtubules. The results revealed a strong association between stiffening of the microtubule network and disease progression. Increases in microtubule stiffening correlated with greater impairments of contractile function and the severity of heart disease in the patients. The group now plan to target the cytoskeleton in these cells, pharmacologically or genetically, to investigate whether reducing microtubule stiffness can improve contractile function of a human heart cell, which would be a powerful demonstration of therapeutic potential.
Future research plans
Dr Prosser’s lab, in collaboration with Prof Ken Margulies at the University of Pennsylvania, is now investigating the use of induced pluripotent stem cells (iPSCs), derived from heart disease patients’ cardiomyocytes, to build organoid models of the heart. These organoids allow cardiomyocyte function to be assessed, including the cytoskeletal structure in human tissue models. Building these organoids from patients with different heart disease types could contribute to a personalized medicine approach to treatment. Organoids could be screened for differing patient phenotypes. For example, some phenotypes may indicate that targeting the cytoskeleton could improve cardiac function, whereas other patient phenotypes could indicate no therapeutic benefit and suggest alternative treatment options.
Dr Prosser explains, “Our work has really benefited from a multidisciplinary approach; leveraging multiple different tools for engineering, access to human samples, iPSC technology and advances in imaging. This is where the best science happens, by combining multiple expertise with advanced tools to probe a really interesting question, real gains are made.”
The use of advanced technologies combined with patient derived cells provides more translational models that can provide better insights into the pathology of heart disease, and ultimately lead to more effective treatments.

