How Gene Therapy is Becoming a Reality: Overcoming Barriers with Innovative Technologies
This new solution could enable large-scale AAV vector production for gene therapy
22 Apr 2019
New data suggests that Corning dissolvable microcarriers could accelerate the field of gene therapy by enabling scalable, high-yield AAV (adeno-associated viral) vector production. Current methods for pre-clinical and clinical trials are limited to two-dimensional (2D) adherent workflows that can be inefficient, time-consuming and labor-intensive, and lack scalability. Keen to find out more, here SelectScience® addresses a panel of expert Corning scientists about existing challenges in the field, and to learn how this unique technology could enable scalable production of viral vectors for gene therapies.

Since the 1990s, over 2,000 clinical trials investigating human gene therapy products have been initiated. For decades, scientists have been attempting to manipulate gene expression for therapeutic effect in inherited diseases – with limited success. However, recent progress in gene delivery and editing technologies has helped to revitalize interest in the field.
The discovery of CRISPR Cas9 is arguably one of the most exciting and emergent scientific stories of the past decade, yet more work is required to fully appreciate potential for inadvertent off-target effects. To date, the most actively investigated in vivo gene delivery entities in humans are recombinant adeno-associated viruses (rAAVs). Comprised of a small protein shell surrounding single-stranded DNA, of approximately 4.8 kb in length, AAVs can traverse cell and nuclear membranes to deliver DNA to the host cell.
Lately, great strides have been made in optimizing these viral particles for patient use. Now, scientists can ensure tissue-specific delivery and are able to improve the resulting expression of proteins through optimization of codon expression, polyA signals, post-transcriptional activity and mRNA stability.
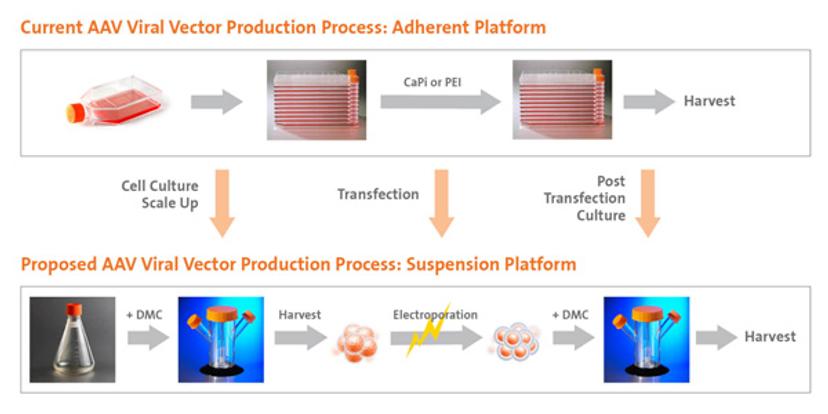
Whilst their safe and effective delivery of DNA to cells has been proven both in vitro and in vivo, as Dr. Zara Melkoumian (technology manager at Corning) explains, the greatest hurdles remain in developing cost-effective and scalable manufacturing processes for AAV production “Moving from flasks to bioreactors is where conventional vector production approaches are at a bottleneck.” Melkoumian continues: The current AAV production process involves culturing adherent cells on plasticware in 2D, followed by CaPi or PEI-mediated transient transfection. This process is time-consuming, labor-intensive and expensive; it requires a high volume of transfection reagents. Ultimately, this is unsustainable for larger scale productions in late-stage clinical trials.”
To combat this, scientists have attempted to develop suspension platforms that are consistent with adherent 2D workflows, but in a scalable, bioreactor environment. In a recent study, Corning has proposed an entirely new process of AAV manufacture using its unique dissolvable microcarrier platform. Omitting the need for a lengthy process for adapting adherent cells to suspension culture the technology could completely transform the AAV manufacturing regimen.
Dr. Jie Wang, leading scientist for the study, underlines the principles of the approach and summarises their key findings: “In this study, we expanded HEK293T cells on the dissolvable microcarriers (DMCs), harvested and transfected them via electroporation, before recovering cells on the DMCs. Amazingly, the complete process requires only three steps, and the results illustrated four key findings. First, our data indicates that the cells expanded on DMCs yielded viral genome particles and infectious titer comparable to those cultured in 2D adherent formats.”
Wang continues: “Second, they produced up to tenfold higher yield than HEK293-F cells in suspension. Both findings confirm the efficacy of DMCs as a platform for AAV manufacture in a format that is conducive to larger scale production. Further, electroporation was an effective transfection approach that was not compromised in 3D culture. Lastly, the dissolution process was much simpler and less harsh to the cells.”
Although DMCs were initially developed by Corning to address challenges in expanding stem cells, the panel explains that their potential application in additional areas, including viral vector production, are now being realized. The unique crosslinked hydrogel microcarrier with specialized surface coatings makes them particularly suited to these delicate applications.
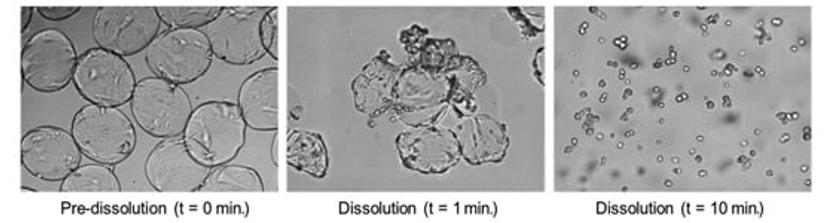
Hannah Gitschier (senior product line manager at Corning) describes why: “Corning’s DMCs are unique because of their dissolvability in combination with surface coatings options — including the synthetic stem cell adhesion promoting surface, Corning Synthemax™ — which enhances the attachment and expansion of delicate stem cells. The technology also enables cells to be harvested gently, to optimize yield and cell quality. The downstream separation of cells from microcarriers post-expansion is a common challenge faced in the industry, but these DMCs can be dissolved in 10-20 minutes with a gentle dissolution solution, reducing the need for a cell – microcarrier separation step in the workflow.”
The need for scalable, robust, and cost-effective viral vector production is on the rise, and this study reveals an exciting new tool that just might help address this increasing demand. By offering a new approach to AAV vector production, this technology may help to accelerate the research and development of gene therapies and treatments with potentially life-saving implications.
Find out more about the Corning® Denatured Collagen Dissolvable Microcarriers.
Disclaimer: Unless otherwise specified, all products are for research use only. Not intended for use in diagnostic or therapeutic procedures. Corning Life Sciences makes no claims regarding the performance of these products for clinical or diagnostic applications.
