How to overcome the RNA delivery dilemma
8 Nov 2024
Dr. David Bunka, Chief Scientific Officer at Aptamer Group
In this guest editorial, Dr. David Bunka shares the benefits of Optimer delivery systems and their potential to overcome targeting issues for improved RNA therapies. David is the Chief Scientific Officer at Aptamer Group, which develops Optimer binders as antibody alternatives for use in a wide variety of applications, including targeted therapies, diagnostics, and research applications.
Why are delivery vehicles needed for RNA therapies?
RNA therapies have emerged as potential game changers in treating diseases, from rare genetic disorders to cancer and autoimmune conditions. These therapies consist of specially designed RNA molecules directed against a gene of interest. The aim is to address imbalances in protein production in the cell/tissue that cause many diseases.
To act as drugs, RNA therapies have to overcome a billion years of evolutionary defences designed to keep invading RNAs from getting to the inside of cells. So, the problem to solve has remained the same – delivery! All other issues with developing RNA as a therapeutic modality have paled compared to the delivery problem. Without an effective delivery system, RNA molecules degrade quickly, fail to reach the target cells, and have limited therapeutic impact as they cannot be taken up by the intended target cells in sufficient amounts. So, the right targeted delivery vehicle can be essential.
All RNA-based therapies, whether small molecules like siRNA and ASOs or larger molecules like mRNA, require delivery vehicles to protect the RNA, direct it to the right site, and ensure it enters the target cells.
What makes RNA delivery so difficult?
The major delivery challenge for RNA therapies breaks down into three hurdles that have to be overcome for effective therapies: 1. Targeting to the specific cell type; 2. Penetrating the target cell membrane, and 3. Ensuring RNA therapy is functional inside the cell.
Going into some more detail: A disease-associated biomarker must be available on the target cells to allow selective targeting of the delivery vehicle. Delivery vehicles bind to cell surface receptors and are internalised by clathrin-mediated endocytosis. We, therefore, need to make sure the delivery vehicle binds to the cell receptor, is internalised, and takes the RNA therapy with it. Once in the cell, escaping the endosome is the next challenge, and many short RNA therapies get stuck in the endosome unable to exert their effect. Only a small amount of RNA needs to escape to be effective, but many therapies remain trapped, limiting their potential.
What solutions are there for RNA therapy delivery?
Researchers have used a variety of nanoparticles to protect RNA therapies in the body. The targeting of nanoparticles has been approached by altering particle components, but many continue to struggle. Adding targeting ligands, such as Optimers or antibodies, to the surface of nanoparticles can help direct them to specific disease-associated biomarkers on target cells.
Alternatively, RNA therapies can be directly conjugated to targeting ligands. This approach is especially effective with smaller RNA therapies like siRNA and ASOs. An example here is the sugar GalNAc, which binds to a protein that is abundant on hepatocytes of the liver. GalNAc has been successfully linked to short RNA therapies or nanoparticles for targeting hepatocytes. Without GalNAc approximately 12% of the total drug in the liver is delivered to hepatocytes, whereas adding GalNAc as a delivery vehicle increases this to approximately 80%. This is a great solution for hepatocytes, but we need equivalent solutions to target the diverse cell types associated with different diseases. We work with partners to help develop delivery vehicles directed to other tissues outside the liver for various therapeutics, including RNA-based therapies.
How do Optimer delivery vehicles address RNA therapy challenges?
Optimers are single-stranded DNA or RNA ligands generated entirely in vitro. Optimers’ lack of reliance on animals or their immune system allows them to target a wider range of biomarkers, including poorly immunogenic ones. During the development process, properties such as selectivity, affinity, and cell internalisation are tailored to ensure Optimers are highly specific and effective as delivery vehicles.
At Aptamer Group, we use two different discovery processes in developing Optimer delivery vehicles: one for specific protein biomarker targeting and another for whole cell targeting. If a specific protein biomarker is known, we start with that protein in our discovery process. We then confirm cell surface recognition in the cell-specific phase of discovery.
If no biomarker is known, we can undertake discovery using whole cells or tissues and screening out binders to non-target cells to drive selectivity. The biomarker that the Optimer recognises can be identified later via proteomics.
Our Optimer delivery vehicle for fibrotic liver was developed using this cell discovery process. This delivery vehicle targets hepatic stellate cells of the liver, the cells that drive scar tissue formation in the liver, to help develop precision medicines for liver fibrosis. Our fibrotic liver delivery vehicle promotes effective siRNA delivery and gene silencing. It is highly selective, binding to HSCs but not other cell types, including kidney, lung, breast, and prostate cells, meaning therapies are targeted with reduced off-site interactions and side effects.
As Optimers are chemically synthesised, the chemistry is fully controlled, allowing site directed conjugation of RNA therapies. This is critical for many of our partners to allow simple and reliable manufacturing and controlled dosing of the final therapies.
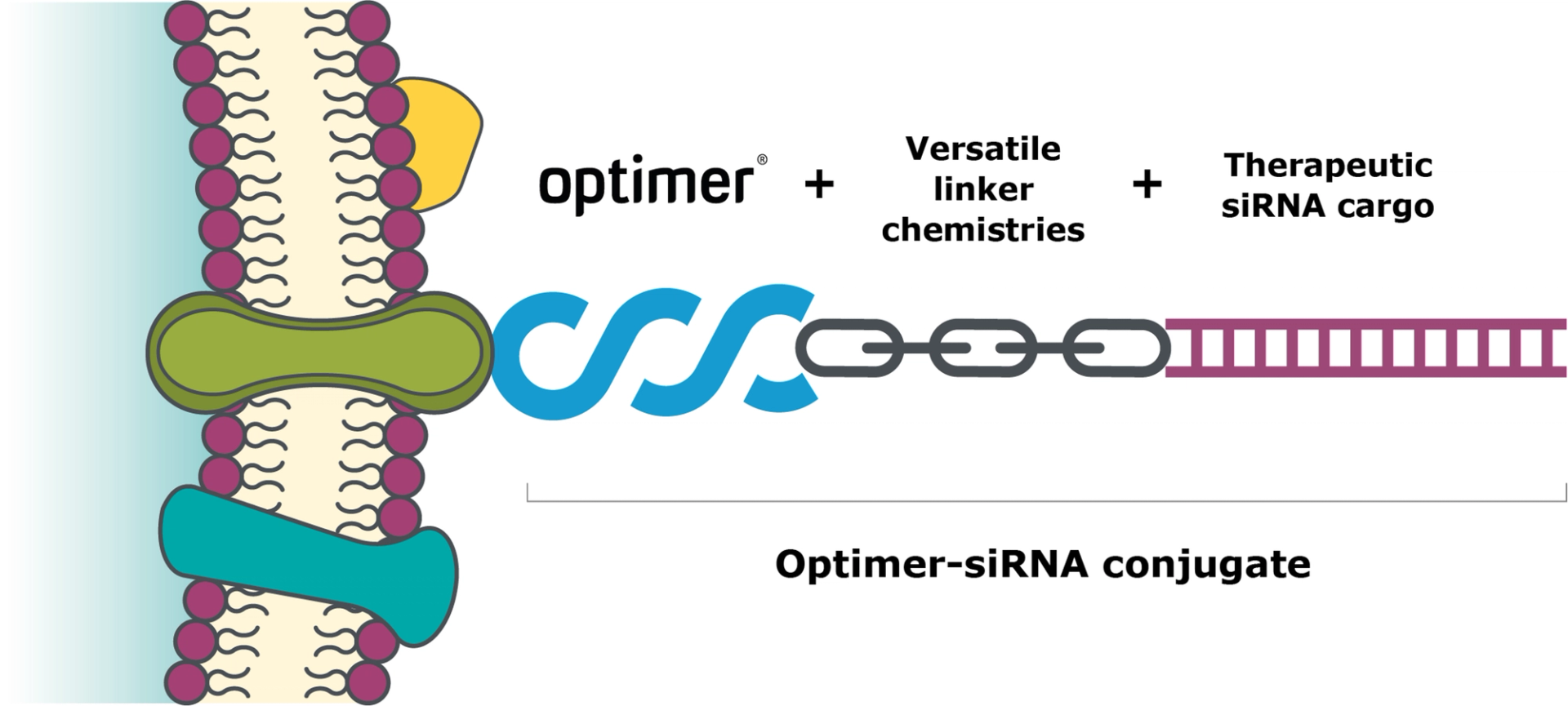
Structure of Optimer-siRNA conjugates from Aptamer Group
What do you see for the future of this field?
This is an exciting field to work in, as researchers across the globe collaborate to find new solutions. You can see the impact of the field from the multiple Nobel prizes awarded for the underlying science over the past few years - RNAi in 2006, mRNA vaccines in 2023, and miRNA just this year. Despite this progress, the lack of viable biomarkers for certain diseases remains a bottleneck. At Aptamer Group, we work with partners to explore new biomarkers and develop Optimer delivery systems tailored to different tissues and cell types.
As more biomarkers are validated, RNA therapies will become increasingly effective, with more precise targeting and delivery options available. Collaborations across academia, biotech and pharma are driving advances for the next generation of RNA therapies to expand treatment possibilities across a broader range of diseases.
Download this free resource to learn about a study that shows how Optimer®-siRNA conjugates effectively silence target genes in fibrotic liver cells for the development of new precision therapies for liver disease.
