Illumina and Kartos Therapeutics partner to develop an NGS-based TP53 companion diagnostic
22 Apr 2021
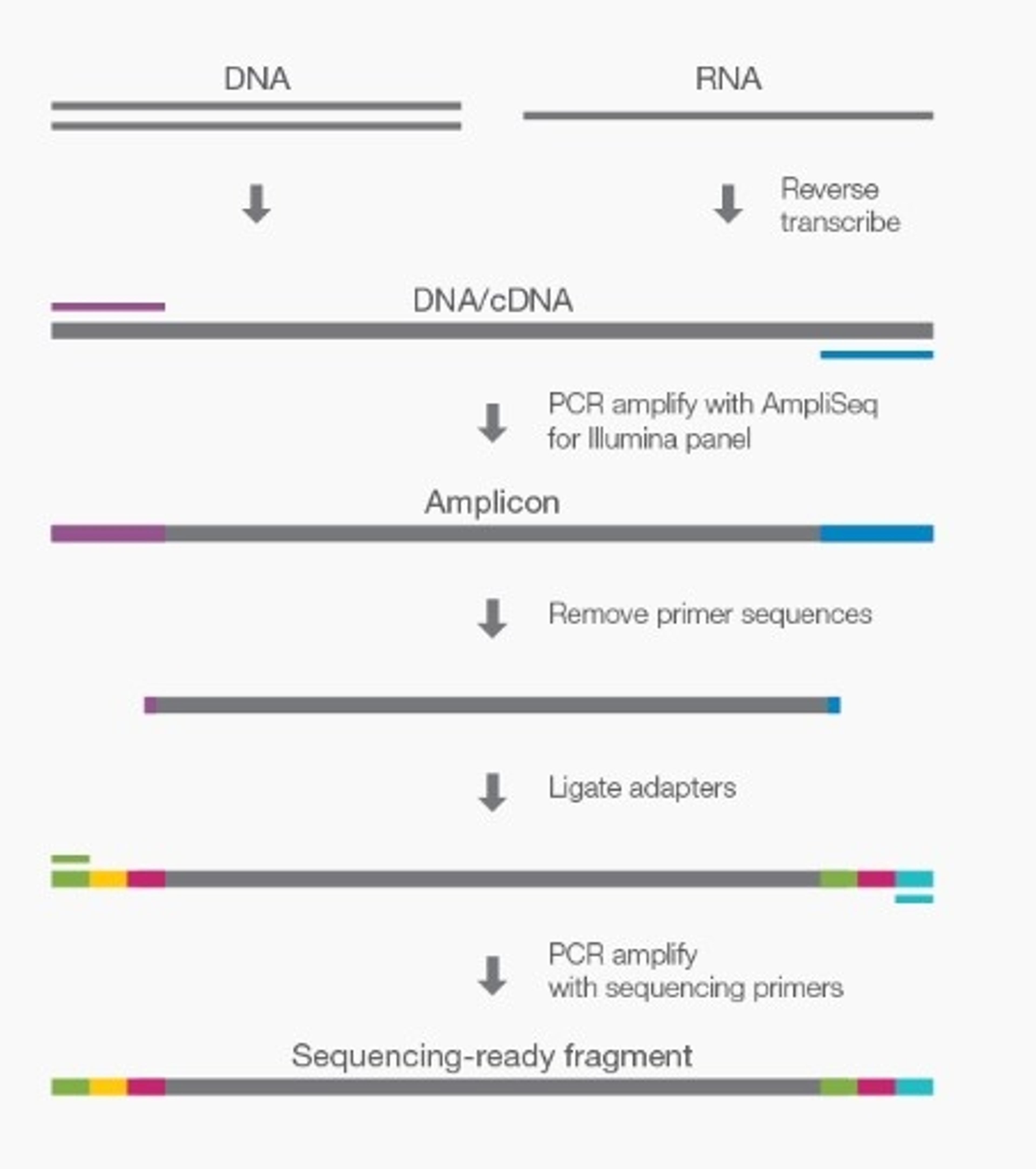
Illumina, Inc. and Kartos Therapeutics, Inc. are pleased to announce a new partnership to co-develop a TP53 companion diagnostic (CDx) based on the content of Illumina’s comprehensive genomic profiling assay, TruSight™ Oncology 500 (TSO 500). This companion diagnostic for multiple hematologic indications will be the first to use TSO 500 with peripheral whole blood as a diagnostic sample type.
“With this partnership, Illumina will expand the TruSight Oncology offerings into hematologic malignancies,” said Phil Febbo, MD, Chief Medical Officer of Illumina. “By leveraging our technology and harnessing the expertise at Kartos, we continue to advance Illumina’s commitment to develop standardized, globally distributable tools for precision oncology.”
The initial focus of the collaboration will be the co-development of multiple CDx claims in blood cancers for Kartos’ KRT-232, a potent and selective oral MDM2 inhibitor that activates p53 to drive tumor cell death in TP53 wild-type cancers.
“Kartos is dedicated to developing novel, targeted therapeutics that meaningfully improve the lives of patients with cancer,” said Jesse McGreivy, MD, Chief Executive Officer and Chief Medical Officer at Kartos. “This partnership will allow us to capitalize on TSO 500 as we explore the expanded use of KRT-232, which offers a unique mechanism to restore the function of p53, one of the most critical tumor suppressor proteins, resulting in apoptosis of malignant cells across a variety of hematologic and solid tumor types.”
The collaboration with Kartos builds on a solid history and varied portfolio of Illumina’s oncology partnerships with industry leaders, with the united goal of advancing cancer diagnostics and precision medicine.
Do you use Illumina products in your lab? Write a review today for your chance to win a $400 Amazon Gift Card>>