Mayo Clinic's Dr Joseph Mikhael Discusses Evolving Multiple Myeloma Clones
Dr Joseph Mikhael addresses the delegation during the 7th International Binding Site Symposium
12 Feb 2016

Dr Joseph Mikhael discussed evolving Multiple Myeloma clones, at the International Binding Site SymposiumDr Joseph Mikhael Dr Joseph Mikhael is a Consultant Hematologist at the Mayo Clinic, and Associate Dean at the Mayo School of Graduate Medical Education, Rochester, USA.
Multiple Myeloma is a monoclonal gammopathy and is associated with clonal proliferation of bone marrow plasma cells. The tumor products produced are monoclonal immunoglobulins and/or kappa and lambda free light chains. Myeloma is a highly variable disease and changes in plasma cell clones during disease have implications for a patient’s therapy and prognosis.
Dr Mikhael, Consultant Hematologist at the Mayo Clinic, US, explained that a recent genomic study of Multiple Myeloma patients had identified three different clonal evolution patterns:
- patients that were genetically stable (no clonal evolution)
- patients showing linear evolution (a single clone dominates during disease)
- patients with a heterogeneous mixture of clones with shifting predominance
It was this last group of patients, with alternating patterns of predominant clones, that Dr Mikhael went on to discuss during his talk.
Case study with a tumor clone switch
One of the patients in the genomic study was evaluated in great detail at seven points during the course of her therapy. This patient was shown to have a classic pattern of extensive IgA intact immunoglobulin Multiple Myeloma disease at diagnosis. She responded very well to her initial treatment, relapsed, was further treated and then went into remission. Further relapses and treatment followed, but with shorter intervals of remission. Both free light chains and monoclonal IgA levels were assessed during her disease. Free light chains were present in only small amounts at diagnosis, however whilst the IgA levels dropped off towards the end of this patient’s disease course, the free light chain levels peaked significantly. This conversion of the dominant monoclonal protein production from IgA to free light chains, suggested that there was some kind of tumor switch in this patient.
In Multiple Myeloma, bone marrow plasma cells produce monoclonal immunoglobulins (such as IgA monoclonal protein) and/or kappa and lambda free light chains.
Importance of monitoring different types of monoclonal protein
If just the IgA monoclonal protein had been monitored during follow up, clinicians may have been misled into thinking that this patient’s disease had been under good control. In fact, the free light chain results showed that her disease was very active.
Dr Mikhael went on to say that this patient’s case was much more complicated than simple free light chain escape. At diagnosis she had one dominant plasma cell clone but also four other minor clones were present. Throughout her treatment these clones alternated in dominance, with each clone type taking dominance at some point. The tests carried out towards the end of her disease showed that it was the smallest, barely detectable clone at diagnosis that ultimately caused her death.
Implications for clinicians
Dr Mikhael highlighted several possible implications for the treatment of future patients. Firstly, multiple clones have variable drug sensitivities and this might be why combination therapies for myeloma have been more successful than single treatments.
Secondly, if a patient has shown resistance to a certain drug, they may not always be resistant at every time point during treatment. Clinicians could consider revisiting therapy courses or combinations that the patient was resistant to earlier in the course of disease.
A further implication was that a minor drug resistant clone was responsible for the patient dying and more work is needed to understand the mechanisms of drug resistant clones.
Finally, the genome in certain high-risk patients has been found to be unstable and the use of DNA-damaging agents, like high-dose melphalan, could be causing further instability. Again, more work is needed to investigate this.
Clonal competition
Dr Mikhael was worried that for a particular myeloma patient a stem cell transplant had caused more harm than good. After standard chemotherapy, this patient was just short of having achieved remission. She then received a transplant and subsequently developed more aggressive disease than she had before the transplant. Dr Mikhael wondered whether the removal of the original clone had allowed a more aggressive clone to rise in its place. If so, should this be a consideration when treating similar patients in the future?
Therapeutic management
As a final note, Dr Mikhael raised a concern that suboptimal therapy dosing could encourage more rapid drug resistance by suppressing one clone but allowing another drug-resistant clone to emerge. In these cases, clinicians must balance the risk of toxicity with the risk of ‘under’ dosing, as well as considering if this is a mechanism of resistance in low dose maintenance.
Multiple Myeloma biomarkers
Multiple Myeloma is increasingly understood to be an exceptionally complicated disease with some patients showing patterns of clonal evolution, tiding and competition. It is important to understand the patterns exhibited by individual patients so that appropriate therapy can be administered. Laboratory tests can help track which plasma cell clones are active.
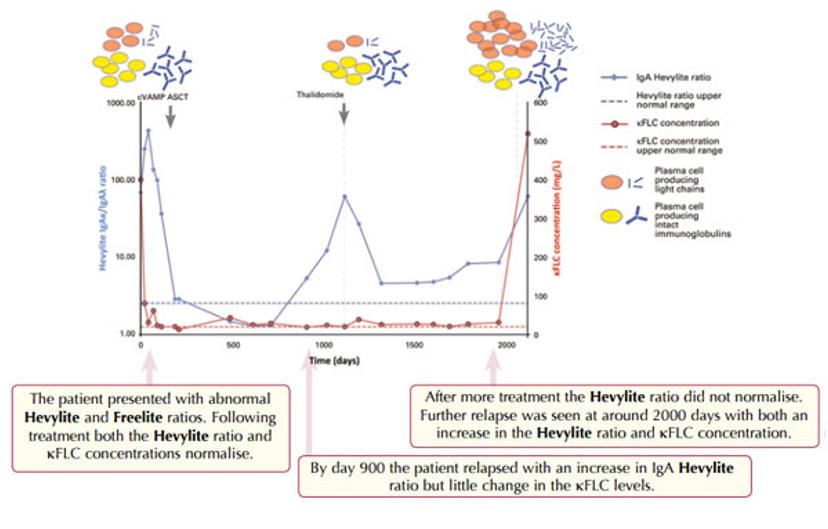
Study of a Multiple Myeloma patient with IgAκ monoclonal proteins plus κ free light chains.
KEY: cVAMP - cyclophosphamide, vincristine, Adriamycin, methylprednisolone. ASCT – autologous stem cell transplant
Freelite and Hevylite are quantitative serum tests that measure two independent biomarkers – Freelite measures kappa and lambda free light chains and Hevylite measures intact monoclonal immunoglobulins (such as IgA). In the monitoring of Multiple Myeloma it is important to use both assays together to capture clonal change, at the point of relapse, in the patient.
View the full presentation here.
