Meet the Sequencing Specialist on a Mission to Revolutionize Cancer Diagnostics with NGS
Dr. Luca Quagliata explains how molecular profiling could transform the way we treat cancer, and how his work is enabling the global adoption of this technology – in the face of clinical challenges and diversity
5 Jun 2019

Initiating change in the clinical space is a monumental challenge, but Dr. Luca Quagliata is a man on a mission with a global vision: to revolutionize cancer therapy with molecular diagnostics. Perplexed by the low response rate to personalized treatment regimens, he has spent seven years leading the molecular pathology and genetics team at the University Hospital of Basel - working to replace single-gene testing panels with upfront next-generation sequencing (NGS) in routine cancer diagnostics.
Now, Quagliata has assumed the role of Global Head of Medical Affairs for clinical next generation sequencing for Thermo Fisher Scientific. In this role, he can leverage his molecular expertise alongside some of the world’s best technologies and services to drive forward his goal of ‘true democratization’ in molecular testing — for improved patient care worldwide. In an exclusive SelectScience interview, we find out more.
Huge potential to improve cancer diagnosis and treatment
Quagliata begins by revealing his personal motivations to transform diagnostics with molecular profiling. “There’s a growing army of small molecules and compounds available on the market to treat cancer; from fusion inhibitors to tyrosine kinase inhibitors, and beyond,” he tells us. “It’s puzzling to me, therefore, that response rates are so low. One of the main reasons for this is the fundamental gap in our understanding of disease biomarkers. Essentially, this means we aren’t administering drugs to the right patients."
We aren't administering drugs to the right patients.
He continues: “Take checkpoint inhibitors for example. When they work, the results are truly outstanding; they’ve revolutionized the way we treat some lung and melanoma cancer patients for example. But, sadly, a large proportion of patients don’t respond – either through innate or acquired resistance mechanisms. Going forward, we need to be able to identify which individuals are susceptible to certain drugs, and this is where NGS comes in. By identifying the broader landscape of somatic mutations, NGS has huge potential to better diagnose and treat patients with cancer, and that’s really the impetus to my work: enabling improved patient care through the technology, services, and knowledge Thermo Fisher Scientific provides.”
Plummeting costs
Since the human genome project reached completion in 2003, sequencing technology has advanced dramatically. The field is now entering an era of so-called ‘third generation’ NGS that is capable of large-scale, real-time sequencing at a fraction of the cost. Fifteen years ago, the Human Genome Project cost just shy of 3 billion dollars, and several years’ worth of research. Now, scientists can run samples for whole-genome sequencing for as little as $3000-$5,000 dollars. Targeted sequencing using small gene panels are even more effective for routine use and can provide more clinically actionable results at a fraction of the cost.
Some of the latest platforms are even capable of detecting structural and copy number variations in genetic material that has previously limited the use of this technology. New techniques also include advanced long-read sequencing that avoids ambiguity from library fragmentation, as well as improved sensitivity in difficult-to-sequence regions – such as GC rich areas and repetitive sequences. Likewise, with the adoption of automated technologies in what’s being called the ‘fourth industrial revolution’, scientists can streamline their NGS workflows right from DNA extraction through to bioinformatic analysis and interpretation.
Consequently, as limitations have been ironed out, performance improved and costs reduced, new fields continue to implement these tools to advance discovery and development. Yet the NGS arena remains uncharted territory for many practicing clinical pathologists. Targeted panels for single genes associated with a clinical phenotype remain the first line of testing for many.
‘To say it’s challenging would be an understatement’
Implementing upfront NGS can provide more informative data to the clinician by assessing a broader spectrum of genetic errors. “That’s the power of multiplexing,” says Quagliata.
He adds: “Another major benefit of NGS is the analysis of liquid biopsies – particularly in patients where it’s not possible to obtain a solid tumor biopsy. Overall, approximately 20% of patients fall into this category; their tumor could be in an anatomically dangerous position, or the patient is at a very advanced stage of the disease and can be quite fragile. Tumor biopsies should still be first-line where possible, but there is mounting evidence to support the clinical utility of liquid biopsies not only as an additional but also as a complementary test.”
But, despite these clear patient benefits, adoption of NGS somehow still remains limited in the clinical space and in major reference medical centers. It is ramping up, however. “When patients are involved, everything is highly regulated and needs to follow a standard operating procedure,” explains Quagliata. “Laboratories often find it difficult to develop validated workflows, harmonize the technology, and achieve accreditation — all within time and staffing constraints. Furthermore, big changes in clinical regimens need to be supported by large multi-center studies; these require many extra resources including local expertise - particularly for data and variant interpretation - and, of course, funding. To say it’s challenging would be an understatement. “Nonetheless, we envision that NGS will have the same uptake in routine testing as immuno-histochemistry. We are actively working to make that happen in the near future with our new generation of NGS solutions.”
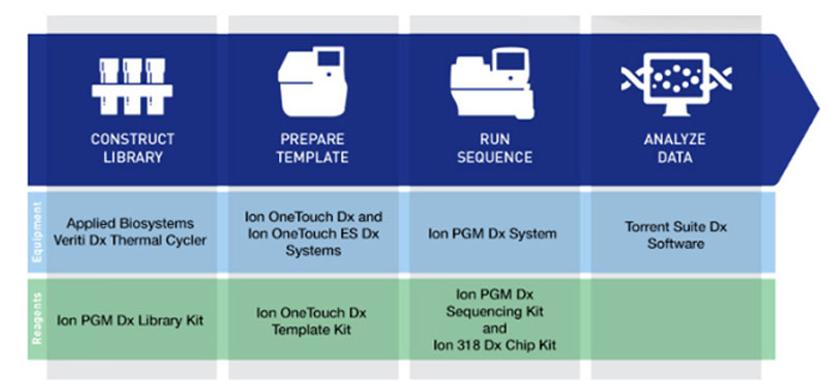
Cutting-edge technology solutions
Clearly, state-of-the-art technology is just a small part of what’s required to implement NGS into routine practice. But the good news is that these challenges have begun to be recognized by the technology providers, who are now offering clinically ready solutions alongside the necessary expertise and support.
This, Quagliata says, was central to his work at the University Hospital of Basel: “After a great seven years, I can proudly say that I was able to transform single biomarker testing into routine upfront NGS, with help from an incredible team, colleagues and vendors such as Thermo Fisher Scientific. This became a really important and practical solution for both clinicians and patients, and now, it needs to be implemented worldwide.”
The global presence of companies such as Thermo Fisher Scientific makes this a real possibility, and experts like Quagliata are now well positioned to directly promote the adoption of NGS internationally. He describes how his primary responsibilities involve developing partnerships with key opinion leaders in academia and pharma, to create utility and validity data that is relevant to each country’s clinical operating standards.
“Alongside the technology, reagents, and assays that Thermo Fisher offers for clinical NGS, we are passionate about generating data to help encourage clinical establishments to adopt these tools, and the journey doesn’t stop there. Our experienced support service team works at the local level to develop in-house solutions that are harmonized, compliant, and streamlined. We make sure that the data we provide is taken up correctly through comprehensive training and ongoing support. Translating study conclusions generated in the context of large clinical trials down to the level of a single patient’s genetic variants can be challenging, and we appreciate that.”
A healthier future
Unequivocally passionate about the power of molecular profiling, Quagliata sees a future of robust and routine NGS services available to all. Beyond that, he predicts that patients will receive therapies to more effectively target the Achilles’ heel of their disease – as more instructive biomarkers, or combinations of biomarkers, are discovered. His personal motives align closely with those of Thermo Fisher Scientific’s, and as his work continues to forge successful relationships between industry and academia, there is mounting anticipation of a rapid and revolutionary uptake of NGS-based molecular profiling that will dramatically improve patient lives.

