Membrane emulsification transforms long-acting injectable manufacturing
A breakthrough approach that overcomes scale-up and consistency challenges in microsphere production
6 Nov 2025
Long-acting injectable microspheres offer numerous benefits to patients and pharmaceutical companies alike. These long-acting drugs release medication steadily over weeks or months, eliminating dosage spikes, improving patient compliance, and ultimately improving patient outcomes. However, the complexity of aseptic manufacturing has historically made these formulations difficult to develop and scale economically.

Camden Cutright, President of Micropore Technologies
To combat this, Micropore Technologies has developed biodegradable polymer microspheres, which are formulated via emulsification. These microspheres are then used for controlled delivery of a variety of active ingredients, including hydrophobic small molecules and hydrophilic proteins.
Camden Cutright, President of Micropore Technologies, is excited about the company’s membrane emulsification platform, an innovative advancement making microsphere manufacturing at scale viable. "People are always looking to improve patient outcomes, and I think packaging small-molecule drugs into microspheres is absolutely something that could work from both a commercial perspective and more importantly improving patient health," he explains.
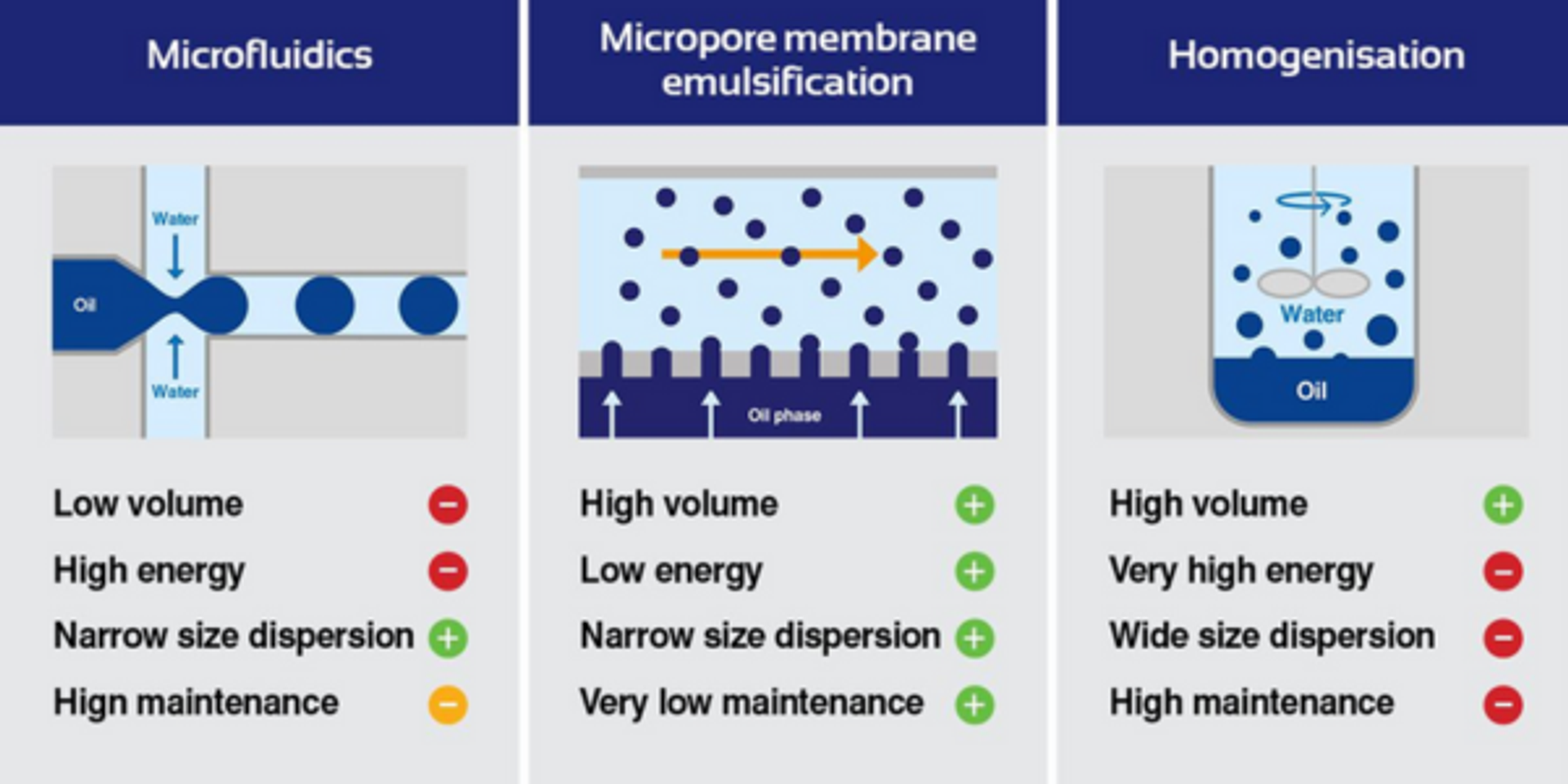
Why conventional homogenization falls short for microsphere manufacturing
The company has moved away from traditional emulsification methods to membrane emulsification, which solves two major problems with the old approach. Firstly, that traditional homogenization is a batch or semi-batch process, making it hard to scale. Second, the energy dispersion inside the homogenizer is hard to control. For long-acting injectable formulations, this inconsistency is particularly problematic because droplet size directly determines how quickly the drug is released into the body.
Membrane emulsification takes a fundamentally different approach, utilizing advanced crossflow membrane emulsification to enable commercial production of mono dispersed single emulsion and double emulsion particles in a reliable, gentle, and energy efficient manner. "By controlling that energy dispersion, you get a very uniform population of droplets. Everything is essentially exactly the same size when it comes out," says Cutright.
During development there have been two key innovations that distinguish Micropore's platform. The first, the development of straight-through pores created by drilling rather than sintering, eliminating blockage and reducing operating pressure. The second breakthrough came in 2016 with the crossflow platform, which transformed membrane emulsification from a semi-batch to a truly continuous process. "It's really a step change in bringing membrane emulsification into an actually viable manufacturing process," notes Cutright.
Precise size control enables predictable drug release profiles
The uniform microsphere size distribution achieved through membrane emulsification directly translates to precise control over therapeutic outcomes. "From a technical perspective, the benefit that Micropore brings is that we get that nice size distribution control. You can make your long-acting injectables to whatever size you want and make them uniformly that size. Importantly you have absolute control over that release profile," explains Cutright.
This predictability is a huge advantage for formulation scientists designing sustained-release products, as it allows them to specify precise microsphere dimensions and confidently predict the resulting pharmacokinetic profile. Uniform size distribution also has a clear real-world effect, with Cutright sharing an example of a veterinary client developing a 90-day long-acting injectable for dogs.
"The client needed to get enough big droplets that would let you last the full three months," he explains. “Their previous method involved filtering out undersized particles, but at tremendous cost, throwing away roughly 60% of everything that they made. Whereas, with our platform, 99% of everything that came through the membrane emulsifier was usable and it didn't require being filtered."
Removing the filtration step offers both clear economic advantages as well as simplifying manufacturing processes. "From a manufacturing perspective, it’s a great benefit. It's a huge benefit in cost, and ultimately it also means one less unit in operation at the end of your process," Cutright points out.
The platform is also designed with aseptic manufacturing in mind – it is small and there are no moving parts or seals. “You don't have to worry about product contamination as a result of the process that you're using," says Cutright.
The same device scales from lab bench to commercial production
Undoubtedly one of the biggest benefits is the elimination of traditional scale-up challenges. Cutright keenly uses a vivid analogy to illustrate why traditional scale-up proves so difficult, "When you're trying to scale up, you think that what you're doing is going from making a cupcake to making a real cake. However, when you are actually doing the process, you realize that every element of the cake is different. The batter is different, the pan is different, the baking time is different, and the temperature is different. You have to change the whole recipe in order to try to get the same cake, just bigger."
Excitingly, continuous manufacturing sidesteps this entirely, as Cutright explains, "The benefit of the continuous process is that you don't have to do that. Once you get your operating conditions to work at the small scale, you're sorted. Your scale-up is ready to go.”
New possibilities for microsphere drug delivery
Looking ahead, Cutright sees substantial opportunity for expanding microsphere applications beyond their current limited presence. "Our platform is a step in the right direction. I think that the control we give to distribution and the ability to save on products improves the economics of all this pretty dramatically. I see a lot of drug development moving towards long-acting injectables in the future," he concludes.
The combination of contamination-free processing, efficiency gains, and seamless scalability offered by membrane emulsification secures its position as an enabling technology for the next generation of long-acting injectable formulations.


