MicroRNA Downregulates Proteins Implicated in Chronic Pain
Dr Maria Lopez deciphers microRNA regulation in pain perception. Read on to learn how she successfully uses multiple primary antibodies from the same host species.
9 Aug 2017

SelectScience interviews Dr Maria José López, a Postdoctoral Fellow at the Interdisciplinary Institute for Neuroscience, Bordeaux, France, to find out more about her research into how microRNA is involved in chronic pain.
Chronic pain is a major public health issue, with almost 1-in-4 people worldwide affected. Chronic pain, often defined as pain which lasts for over three months, can sometimes be triggered by an injury or illness such as cancer, but can also have no obvious cause. Many patients don’t respond to painkillers, leading to reduced mobility and a likely reduction in the patient’s quality of life.
Dr Lopez is working as part of the Central Mechanisms of Pain Sensitization Group, a research group which aims to uncover the pathway dysfunctions which are responsible for chronic pain.
“The aim of my work is to decipher the molecular mechanisms of cancer-related chronic pain,” says Dr. Lopez. The hope is that the team can then manipulate these pathways in order to avoid the establishment of chronic pain and to inform the development of effective painkillers and therapeutics for the disease.
Elucidating the role of microRNA
One of the molecular mechanisms Dr Lopez is focusing on is the regulation of the pain pathway by microRNA (also known as miRNA). MicroRNAs — small, non-coding RNAs which regulate gene expression through post-translational inhibition and degradation of mRNA — were first identified as playing a role in inflammatory pain in 2007, and ever since they have been implicated in the regulation of almost all pain pathways.
“Our protein of interest is called synaptopodin, an actin-binding molecule involved in the formation of the spine apparatus,” says Dr Lopez. “It has been shown that synaptopodin-deficient animals do not form spine apparatus and demonstrate deficits in synapse function. Therefore, this protein plays a critical role in synaptic transmission and, thus, in pain processing,” explains Dr. Lopez.
Investigating microRNA regulation using immunofluorescence
Dr Lopez investigates the role of microRNA in the regulation of synaptopodin using a range of techniques including qPCR, microRNA screening, Western blotting and immunofluorescence. In her most recent paper (unpublished, submitted to Scientific Reports), Dr Lopez utilized immunofluorescence to investigate whether microRNA was downregulating the expression of synaptopodin in mouse neurons.
“We injected mice intrathecally (into the spinal cord) with a plasmid containing our microRNA against synaptopodin mRNA. The plasmid also contained a gene for DsRed, a marker protein,” explains Dr Lopez.
We decided to try the Fab fragment affinity-purified secondary antibodies to cover one primary antibody and present it as a different species.
Dr Lopez and her team then needed to determine whether synaptopodin was downregulated in these microRNA and DsRed-expressing neurons, by carrying out immunostaining on prepared spinal cord slices. However, she encountered a problem.
“To identify the neurons which were expressing the microRNA, we used an antibody against the co-expressed DsRed. We also used another primary antibody against synaptopodin to assess the protein’s expression levels. The problem arose as both these primary antibodies came from the same species: rabbit. There were no other suitable antibodies on the market, and it was not possible to make them in-house,” explains Dr Lopez.
Double-labelling with Fab fragments
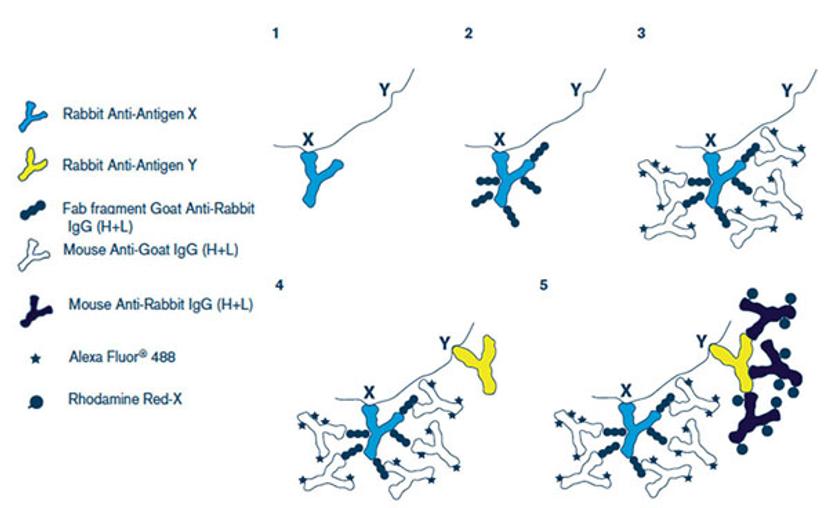
To resolve this, Dr Lopez used Fab fragment affinity-purified secondary antibodies from Jackson ImmunoResearch. These enable double labeling of primary antibodies from the same host species.
“We decided to try the Goat Fab Anti-Rabbit to cover one primary antibody and present it as a different species. We followed the protocol provided by Jackson ImmunoResearch and it worked perfectly, allowing us to detect both primary antibodies simultaneously,” says Dr Lopez.
Monovalent Fab fragments only have a single antigen binding site, making them useful in many immunostaining applications. They can be used to reduce background staining, as an alternative to chemical conjugation, or, as Dr Lopez used them, to enable double labeling of antibodies from the same species.
Dr Lopez was then able to use two different fluorescent-labeled secondary antibody conjugates to quantify both microRNA and synaptopodin levels in the neurons. “The results showed that in DsRed positive cells, the fluorescence signal from synaptopodin was decreased in comparison to the control group – mice injected intrathecally with a GFP plasmid,” explains Dr Lopez. These results support the hypothesis that this microRNA is downregulating synaptopodin and could perhaps be involved in the development of chronic pain.
Hopes for future therapies
Dr Lopez hopes that this research will contribute to a better understanding of the role of microRNA in pain and perhaps uncover ways in which they can be manipulated to prevent the establishment of chronic pain.
Drugs which either increase or decrease specific microRNAs in the nervous system could be a potential therapy option for chronic pain patients. Potential strategies which are being investigated include microRNA mimics, photo-activable microRNAs and microRNA sponges. However, there are many challenges for the future development of microRNA as a therapy, which include: finding the best microRNAs to target, perfecting a delivery method and finding ways to limit potential side-effects.
Find out more about Fab Fragment Affinity-Purified Secondary Antibodies from Jackson ImmunoResearch.
Do you use Jackson ImmunoResearch products in your research? Write a review for your chance to win an Amazon voucher worth $400 or an iPad.

