New Liquid Biopsy Test Can Monitor Cancer From a Single Drop of Blood
Circulogene’s core technology enables cell-free DNA monitoring in oncology
1 Jan 2017
Dr. Chen-Hsiung Yeh, Circulogene’s Chief Scientific Officer Circulogene Theranostics Circulogene is a leader in molecular theranostics. The company, based in Birmingham, Alabama, is founded and operated by leaders and scientists from the diagnostics industry.
Our test results will help oncologists to choose the right treatment, for the right patient, at the right time
Dr.
Chen-Hsiung Yeh
Circulogene Theranostics
Circulogene Theranostics has launched into the liquid biopsy market with a test that is able to enrich circulating cfDNA from a single drop of blood to monitor cancer therapy. Sonia Nicholas, SelectScience®’s Clinical Editor, spoke to Dr. Chen-Hsiung Yeh, Circulogene’s Chief Scientific Officer, to find out more.
Signature of a tumor
SN: Could you start by telling us what cell-free DNA is?
CY: Cell-free DNA (cfDNA) is DNA molecules that have been released from cells. When cells die, they release their intracellular content, including DNA, into the bloodstream. The concept of cell-free DNA has already been applied in non-invasive prenatal testing for years. In the oncology arena, tumor cells release their DNA into the circulation even more rapidly, due to their high turnover rate. These DNA fragments represent a genetic footprint or fingerprint that the tumor cells have left behind.
SN: What is cfDNA testing used for?
CY: The peripheral cfDNA molecules contain genomic alterations specific to tumor cells. This provides us, for the first time, with a non-invasive, real-time and longitudinal way (as opposed to invasive, one time-point, single-site sampling in traditional tissue biopsy), to monitor tumor heterogeneity and clonal dynamic evolution. Clinically, the information gathered from cfDNA can guide clinicians in treatment decision-making, monitoring treatment efficacy, monitoring minimal residual disease, drug resistance, recurrence, and metastasis.
Perfecting the technology
SN: What are the limitations of cfDNA testing using current methods?
CY: The requirement for large sample volumes, low yield, and labor-intensiveness are all major obstacles for the routine application of cfDNA-based testing in the clinic. The reason for this is that all companies are using the same methodology to extract cfDNA, i.e. silica matrix-based (either spin column or magnetic beads). Unfortunately, we have known for years that this extraction process loses a lot of material due to binding, washing, and elution steps. For academic research projects, it doesn’t really matter, but in a clinical setting, loss of 70-80% of starting materials means your end results will be greatly compromised, leading to inappropriate patient management. The impact is tremendous, especially in targeted therapy for cancer patients.
Nevertheless, all liquid biopsy companies are still working towards perfecting their own technologies to selectively enrich or amplify tumor-specific cfDNA from a dominantly normal population. Imagine that their starting materials are still those “partial” DNA molecules that survived after the extraction/isolation process. As a result, no matter how sensitive their technology is (downstream), they cannot detect cfDNA already lost during sample preparation step (upstream). This is the key point that everyone in this field overlooked or even ignored. They don’t know what they’re missing. On the other hand, at Circulogene we have focused on this very first step, to make sure that we capture all of the cfDNA we can.
SN: Please could you describe how your proprietary method of analyzing cfDNA works?
CY: We reasoned that in circulation, the released cfDNA fragments are not “naked”, but complexed with proteins and lipids. This special property not only shifts their buoyant density to values much different from that of bulk chromatin, free DNA or protein, but also protects the corresponding cfDNA from attack by circulating nucleases. Density fractionation allows us to selectively separate and concentrate cfDNA fragments based on their protein/DNA ratio, i.e., euchromatin (active genes) vs. heterochromatin (inactive genes).
Our concentration method enables advanced genomic analyses (e.g., next-generation sequencing) directly from droplet volumes of plasma or serum (as low as 20 uL via a simple finger-stick). This protocol can be applied to a broad range of clinical genetic tests, with the advantages of minimal sample volume, maximal yield, streamlined and automatic workflow with reduced costs and turnaround time. Most importantly, taking into account the fact that the vast majority of the human genome doesn't code for proteins (approx. 98%), density-based enrichment technology makes perfect sense for cfDNA clinical application over the other random, blind-extraction methods.
SN: What are the potential benefits of this technology in cancer diagnostics/therapy?
CY: The takeaway message is this – our test results will help oncologists to choose the right treatment, for the right patient, at the right time.
More sensitive and accurate test results due to no loss in starting materials.
Quicker turnaround time: 5-7 days (due to high-degree of automation) compared to tissue biopsy 3-6 weeks, and current liquid biopsy 2-4 weeks.
Cheaper than traditional tissue biopsy (on average $15,000) and current liquid biopsy tests (on average $5,000), reducing patient and health care burden.
Non-invasive, real-time, longitudinal monitoring vs. invasive, out-of-date, single-point data (tissue biopsy).
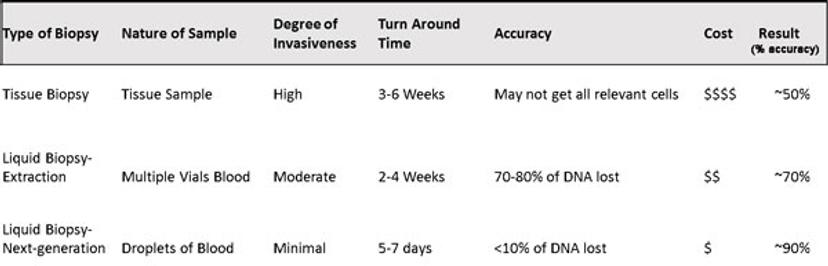
Comparison of different types of biopsy - Table provided by Circulogene Theranostics
SN: Can you describe what research studies you are working on at the moment?
CY: We’re actively collaborating with several academic research institutions to further validate and implement our technology. We will have more clinical validation data available next year.
SN: Is the test approved for clinical use yet?
CY: Yes, it is approved. Circulogene is a CLIA-certified clinical laboratory, we are currently processing clinical samples.
About Dr Chen-Hsiung Yeh
Dr. Chen-Hsiung Yeh has over 20 years’ experience of drug discovery and molecular diagnostics in biotech, pharmaceutical, and diagnostic industries. He has held leadership roles at Pfizer, Response Genetics, Quest Diagnostics, and most recently Atherotech Diagnostics. Chen is a pioneer in the liquid biopsy field and the inventor of Circulogene’s core technology.
