New methods to rapidly evaluate virus-neutralizing antibodies
Learn how high-throughput, real-time cell analysis can accurately detect and quantify virus-neutralizing antibodies and see how this technology is advancing COVID-19 research
8 Oct 2020
The COVID-19 pandemic is driving the need for rapid development of effective vaccines and therapies. A key starting point to approach the control of viral infections, like COVID-19, is to understand the immune response and the antibodies that confer immunity. The analysis of virus-neutralizing antibodies that are produced as part of the natural immune response provides insight into targets for vaccine or therapeutic antibody development.
Virus-neutralizing antibody assays are a key tool in virology and aid the identification of effective antibodies against a virus. However, current methods can be time consuming and the information obtained is limited. In this SelectScience article, Yama Abassi, Ph.D., Director of Business Development at Agilent Technologies, discusses next-generation methodology for the analysis of virus-neutralizing antibodies and explores how Vanderbilt Vaccine Center researchers have implemented the Agilent xCELLigence RTCA HT system for high-throughput screening of SARS-CoV-2 antibodies. Additionally, with the latest xCELLigence RTCA eSight, researchers are able to combine both real-time impedance and live-cell imaging to capture even more information from a single experiment.
Virus-neutralizing antibodies
Virus-neutralizing antibodies play a key role in protection from viral infection. Produced as part of the adaptive immune response, these antibodies bind to specific antigens on the virus surface, which block the virus from interacting with the host cell. By neutralizing the ability of the virus to bind to the host cell, viral replication and infection is consequently prevented.
In the case of SARS-CoV-2, one antigen of interest is the spike glycoprotein on the virus surface1. The S1 subunit of this protein contains a receptor-binding domain (RBD) that enables the virus to infect host cells by binding to the ACE2 receptor present on the host cell surface. The S1 RBD is highly antigenic, triggering an immune response and the generation of immunoglobulin G (IgG) antibodies against the RBD.
Identifying the most effective virus-neutralizing antibodies, therefore, holds promise for the development of therapeutic strategies against viral infections, such as vaccination and immunotherapy for the prevention of infectious disease.
The severity of the COVID-19 pandemic demands accelerated timelines for the development of such therapies against SARS-CoV-2, and there is a need for innovative technologies that shorten experimental time to lead discovery.
Challenging the laboratory gold standard assay
As many viruses use the lytic life cycle, the analysis of host cell health can be used to determine viral activity. Monitoring cytopathic effects (CPE) - the changes observed to host cells upon viral infection – is typically performed by plaque reduction assays.
The current laboratory gold standard assay, the plaque reduction neutralization test (PRNT), detects the effectiveness of virus-neutralizing antibodies2. The PRNT assay measures the ability of the antibody to reduce the number of plaque units formed when cultured in various dilutions with a confluent monolayer of host cells, and plaques are subsequently counted by visual inspection.
While this test is the most widely-accepted approach, there are several pain points for researchers performing the technique. Firstly, the method is time-consuming, taking numerous days, or even weeks, to complete and requiring multiple handling steps. As with other single-endpoint assays, information obtained by PRNT is limited – for example, the method is not able to indicate the onset of the cytopathic effect or the kinetics of virus-mediated cytotoxicity. In addition, consistency between experiments can vary, with manual counting of plaques by microscopy subjective to the user and many factors – such as cell type, cell density, as well as viral strain, serotype, and mutations – influencing plaque formation rates and size, making optimum assay endpoint essential. Failure to do so can result in the inaccurate calculation of viral titer and lytic activity.
xCELLigence Real-Time Cell Analysis
xCELLigence instruments use patented microplates containing gold biosensors to detect subtle changes in a miniscule electric current as it passes through adherent-cells. By measuring impedance of this current, quantitative kinetics of viral cytopathic effects can be quantitatively measured over time.
Faster analysis of virus-neutralizing antibodies
An alternative to the traditional PRNT assay, Agilent Technologies’ xCELLigence Real-Time Cell Analysis (RTCA) workflow enables rapid evaluation of virus-neutralizing antibody studies. Designed to track virus-induced cytopathic effects in real-time, the technology enables the accurate detection and quantification of neutralizing antibodies throughout the virus lifecycle. “The initial rate of infectivity of the virus, which can be measured by xCELLigence system, and its inhibition by neutralizing antibodies can provide a fairly accurate assessment of virus neutralization activity, precluding the need to wait for several days as required by the PRNT assay,” explains Abassi. The technology also overcomes the subjectivity of the visual interpretation of PRNT assays, providing more precise and reproducible results.
With models compatible with 96- and 384-well formats, as well as disease relevant and primary cells, the system is designed to maximize data acquisition and streamline virology experiments. “The simple screening assay consists of seeding cells in specialized microplates, adding pre-incubated virus-antibody mixture to the seeded cells, and letting the xCELLigence system, which is housed inside a CO2 incubator, automatically acquire data in real-time,” Abassi describes. A high-throughput mode can be used to screen for potent antibodies at a given antibody concentration, followed by an antibody-dose response assay to rank order the antibodies in terms of their potency.
“Our users get automatic results through the xCELLigence RTCA software, which can be used to quantitatively evaluate antibody efficacy by IC50. Additionally, this workflow does not require agar, dye, fixative, or labels, which greatly reduces workload and manual handling of samples to minimize risk of exposure to infectious material,” Abassi shares. The virus-neutralizing antibody screen can also be used for monitoring the efficacy of vaccination, and for elucidating the kinetics of virus resistance emergence.
Real-time cell analysis technology in action: Advancing therapeutic antibodies for SARS-CoV-2
The technology has already been implemented in the quest for COVID-19 therapeutics by researchers at the Vanderbilt Vaccine Center, Vanderbilt University Medical Center. The study, by scientists in the James E. Crowe lab, published in Nature Medicine, details a rapid high-throughput workflow for the isolation and profiling of SARS-CoV-2 neutralizing monoclonal antibodies utilizing the xCELLigence RTCA HT Analyzer3. The platform developed enables the isolation of hundreds of human monoclonal antibodies (mAbs) against the SARS-CoV-2 spike (S) protein, which were then screened using the xCELLigence RTCA technology to quantify and rank mAbs with full or partial neutralizing activity. Abassi shares: “The data obtained from xCELLigence system was critical to advancing antibodies for therapeutic use.” The authors confer the success of the workflow in defining the SARS-CoV-2 S protein receptor-binding domain as a target for vaccine and therapeutic antibody development.
More information from one experiment
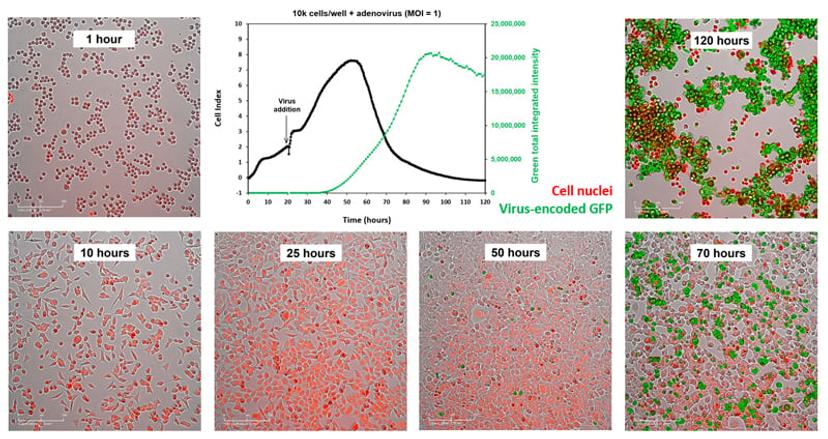
The latest offering in the xCELLigence RTCA product line, the xCELLigence RTCA eSight, enables researchers to gain extensive phenotypic information from virus-neutralizing antibody assays. This system couples real-time impedance with live-cell imaging to provide sensitive and information-rich measurement of cytopathic effects that span the entire viral life cycle. “With one experiment, the eSight provides two simultaneous readouts and reduces workload by using the same microplate and treatments to gain multiple perspectives,” Abassi explains.
The eSight’s brightfield imaging provides both a qualitative and quantitative assessment of cell morphology, confluence, and cell aggregation under various treatment and assay conditions. With three distinct fluorescence channels and various live-cell imaging reagents, the eSight can also reveal cellular details such as barrier function disruption, activation of host cell anti-viral pathways, and apoptotic death. The system is even able to detect the formation of syncytia – the enlarged multi-nucleate cells caused by the fusion of virus-infected cells with neighboring cells.
Further characterizing the immune response and virus behavior
The immune response to viral infection is complex and multi-faceted. In addition to the humoral immune response mediated by the B cell secretion of virus-neutralizing antibodies, there is a cellular response mediated by T cells, which is equally important and can also confer lasting protection and immunity against viral infections. The T cell response has the ability to identify viral-infected cells and either eliminate them directly or recruit other arms of the immune response for elimination of virally infected cells. In addition to virus-neutralizing antibody screens, the xCELLigence RTCA system can be used to test the recognition and elimination of virally infected cells by T cells, providing an accurate measure of the potency of cellular immunity.
The system can also be used to perform a number of other applications, including determination of virus titers and virus quality, as well as in the study of oncolytic viruses and the efficacy of antiviral drugs and virucides.
Learn more about virology applications of Agilent Technologies’ xCELLigence RTCA technology in this downloadable handbook.
For Research Use Only. Not for use in diagnostic procedures.
Agilent products are not approved, marketed, or validated for COVID-19 testing, diagnosis, treatment, or mitigation. Agilent has not validated a product to detect the novel coronavirus or antibodies.
References
Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, Guo L, Guo R, Chen T, Hu J, Xiang Z, Mu Z, Chen X, Chen J, Hu K, Jin Q, Wang J, Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020
Timiryasova TM, Bonaparte MI, Luo P, Zedar R, Hu BT, Hildreth SW. Optimization and validation of a plaque reduction neutralization test for the detection of neutralizing antibodies to four serotypes of dengue virus used in support of dengue vaccine development. Am J Trop Med Hyg. 2013
Zost SJ, Gilchuk P, Chen RE, Case JB, Reidy JX, Trivette A, Nargi RS, Sutton RE, Suryadevara N, Chen EC, Binshtein E, Shrihari S, Ostrowski M, Chu HY, Didier JE, MacRenaris KW, Jones T, Day S, Myers L, Eun-Hyung Lee F, Nguyen DC, Sanz I, Martinez DR, Rothlauf PW, Bloyet LM, Whelan SPJ, Baric RS, Thackray LB, Diamond MS, Carnahan RH, Crowe JE Jr. Rapid isolation and profiling of a diverse panel of human monoclonal antibodies targeting the SARS-CoV-2 spike protein. Nat Med. 2020


