New Optical Method “Cuts” and Examines Cancer Biopsies During Surgery
Dr J. Quincy Brown explains how optical methods examine fresh tumor biopsies to determine the tumor margins during organ removal surgery
27 Jul 2017
As a surgeon removes a tumor from a patient, he or she goes through a decision-making process that will affect the patient’s health and lifestyle after surgery — how far do I cut to get all of the tumor out? When it comes to determining the difference between a successful resection surgery versus a patient who might need repeat procedures, a key factor is often the edge of the tumor mass known as the tumor margins. SelectScience® speaks with Dr. J. Quincy Brown, Assistant Professor of Biomedical Engineering at Tulane University, who improves the accuracy and speed of establishing tumor margins on fresh biopsies alongside surgeons in an operating room, using optical methods that require no physical cutting of the tissue.
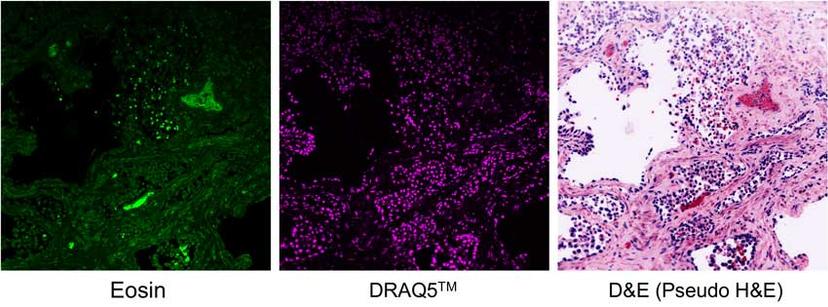
Optical sectioning via fluorescence microscopy eliminates physical sectioning of biopsy tissues. Show here, prostate tissue stained with eosin (left) and DRAQ5TM (middle). The D&E panel (right) is the combined and recolored image to simulate the traditional H&E staining. (Image courtesy of the Brown lab, Tulane University)
Tumor margins: Where to stop surgical removal?
"When we were talking to surgeons, we became interested in this problem of tumor margins during surgery,” says Dr. Brown who has previous experience in optical biopsy where he would help radiologists determine tumor locations inside the body using optical spectral probes. He became interested in studying tumor margins when he learned of its serious implications. “In breast cancer, for example, when a tumor is removed, 20–70% of the time, it’s not possible to remove it completely. The patients usually have to come back for additional surgery and their risk of mortality goes up. This is a big problem which costs time, money, pain and above all, patient anxiety."
The new 'D&E' method replaces the H&E staining for cancer biopsy histology The Brown lab has developed an optically compatible method using DRAQ5TM from Biostatus coupled with eosin, which substitutes the conventional H&E method. This new method helps examine fresh biopsy samples to check for tumor edges after surgical removal.
The Brown lab focuses on tumor margins in prostate cancer where, unlike some other forms of cancer, it’s impossible to repeat the resection surgery. “The prostate is in such a position in the body that it is surrounded by vital organs that are crucial for post-operative functions like bladder control and sexual activity. Surgeons don’t want to disturb those,” says Dr. Brown. “As tumors become more advanced, they expand and extend beyond the prostate into the surrounding vital structures. So, the surgeons have a challenging task — they have to try to figure out precisely where the tumor edge is. They want to cut as close to the tumor as they can because with every millimeter of tissue they remove beyond the prostate, they’re eliminating post-operative function for the patient.”
In a study published in Scientific Reports, the team used structured illumination microscopy (SIM) to directly examine the surface of the prostate taken out during surgery. “As opposed to a single layer of cells in vitro or a sliced piece of frozen tissue section, we’re looking at tissue that’s thick and fresh out of the body, basically the whole organ. We need to cut it somehow. Rather than physically cut them, we optically cut it,” explains Dr. Brown.
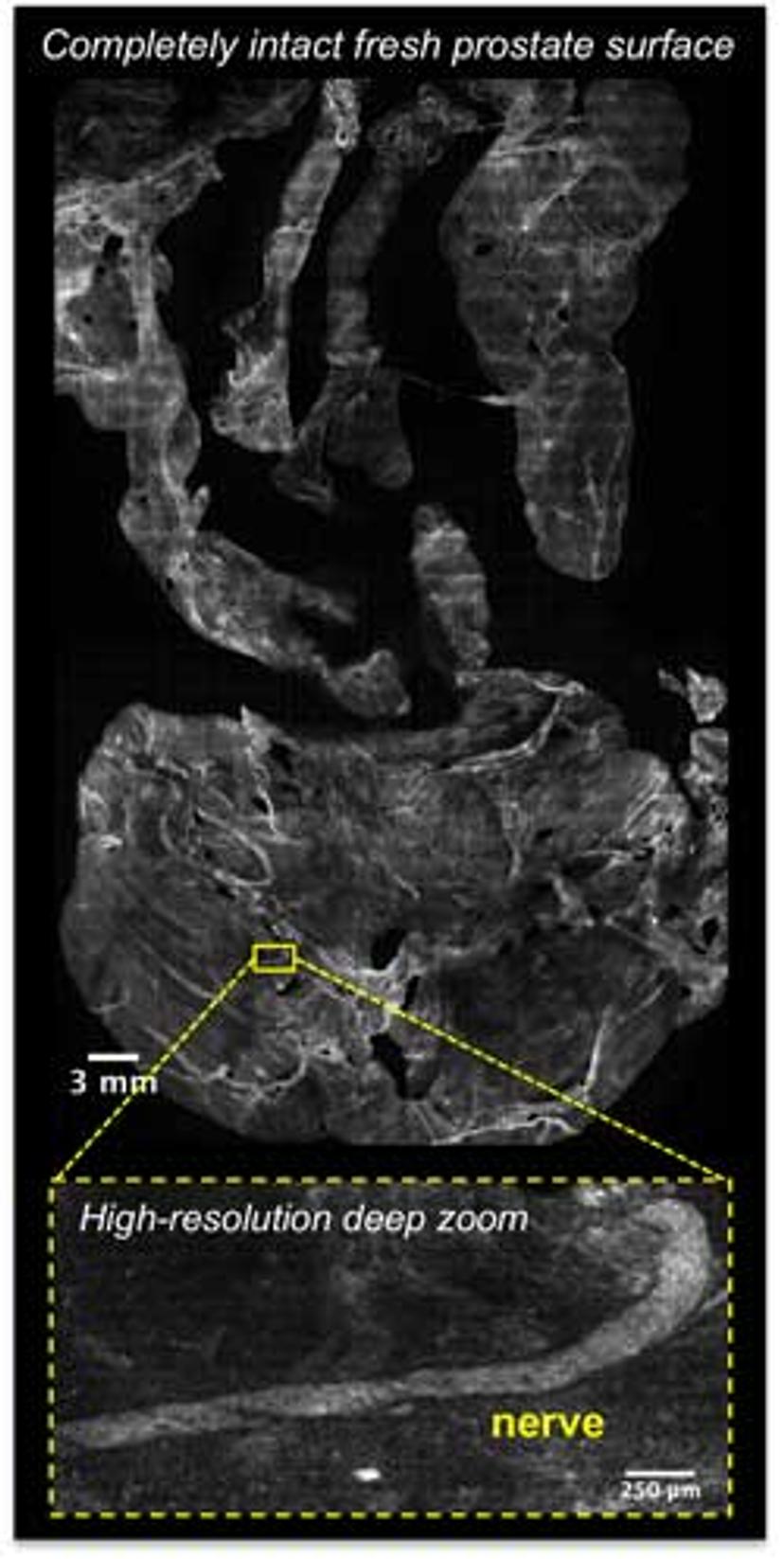
A large Google Maps-style image of the entire surface of a freshly removed tumor can be searched for remnants of tumor at the edge, providing feedback to surgeons. (Image courtesy of the Brown lab)
The benefits of using advanced optical microscopy to “cut” fully intact tumor samples, instead of the conventional, labor-intensive ‘freeze and section’ approach is that the former is very fast and can cover a large amount of tissue volume in a very short amount of time, a change that is readily welcome in the clinic.
‘Do I see any cancer on the edge of this specimen?'
Dr. Brown describes how he works in collaboration with surgeons and clinical pathologists: “During surgery, the surgeon can leave much of the normal tissue adjacent to the prostate to maximize postoperative function. They then give us the resected organ. We dip the organ in dyes, put it on a flatbed scanner, and take very large Google Maps-style images of the whole surface. Then the pathologist, who is sitting in their office, gets the images online. They take a look and decide, ‘Do I see any cancer on the edge of this specimen?’ If they do, they call the operating room and tell the surgeon, ‘Here is where I see residual tumor. You need to go back and cut more tissue.’”
To examine a tumor optically, it is necessary to use dyes or markers to view underlying cells. The team’s first study on whole prostate imaging used a single dye, which gives a grayscale image. However, the conventional method employs the gold standard hematoxylin and eosin (H&E) staining on thin sections of tissue followed by examination by a pathologist, which gives a two-color image that pathologists are trained for years to recognize. To advance their novel optical ex vivo imaging, Dr. Brown’s lab tried to replicate the H&E staining but using fluorescence-friendly dyes. The new method, named D&E, which stands for DRAQ5 and eosin, involves incubating the fresh biopsy tissue in DRAQ5, a cell-permeant DNA probe, and the standard eosin dye. “The ‘E’ in H&E is naturally fluorescent. So, we were searching for a nuclear marker that is spectrally separated from eosin. We came across DRAQ5, which, due to its excitation and emission spectrum in the far-red region of the spectrum, is a perfect combination with eosin. Furthermore, it is very specific for DNA and bright,” says Dr. Brown, who published the new D&E findings in a PLOS One publication.
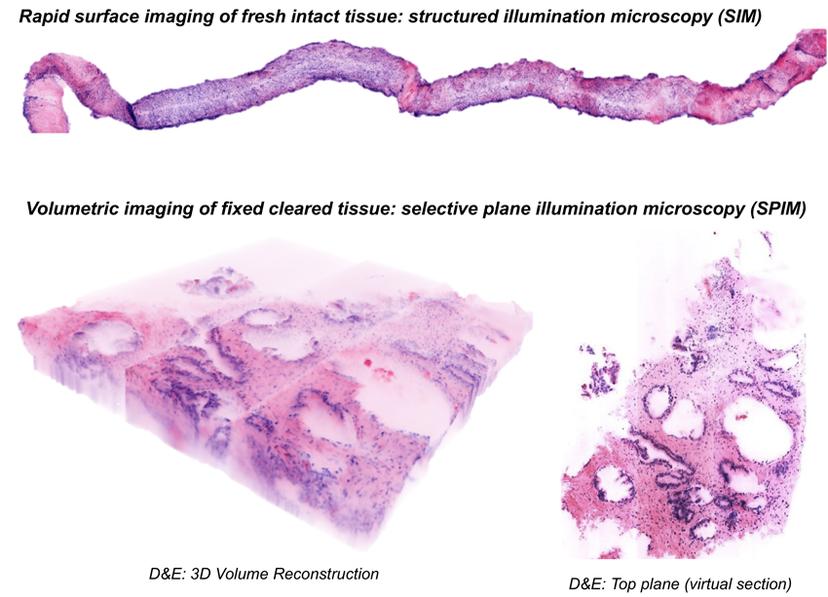
D&E (DRAQ5 and eosin) is a general method that can be combined with different types of optical sectioning microscopy for different applications such as, structured illumination microscopy (SIM) (top), and selective plane illumination microscopy (SPIM) (bottom). (Image courtesy of the Brown lab)
“Instead of H&E we now have D&E that can be paired with optical methods such as SIM during a surgery for imaging the entire surface of freshly resected tumors, or with selective plane illumination microscopy (SPIM) for imaging tumor biopsies in 3D. With just a 1- to 3-minute incubation, we can stain an entire biopsy tissue volumetrically with DRAQ5 and eosin to get a high-fidelity match to H&E,” adds Dr. Brown.
“We’re giving surgeons a safety net,” he concludes. “Hopefully, what that will do is enable more patients to benefit from organ-sparing or nerve-sparing surgeries that will maximize post-operative functions and will prevent recurring tumors.”
Find out more about the DRAQ5 and other Biostatus fluorescent probes.
Do you use DRAQ5 in your research? We want to hear from you! Share your expertise here.