Organoid-based 3D model of human glioblastoma: Recreating brain tumors in a dish
Discover how a brain organoid core facility is applying its glioblastoma model towards developing patient mini-brains
7 Apr 2021
Glioblastoma, the deadliest of primary brain tumors in adults, has a median survival of only about 15 months, with less than 6% of patients surviving past the five-year mark. The urgent need to fully understand the cancer biology of glioblastoma is thwarted by the lack of a suitable disease model that mimics the cerebral microenvironment.

In recent times, cerebral organoids, the self-assembling three-dimensional cellular models derived from stem cells, have opened up the possibility of replicating the brain’s milieu to closely examine and study glioblastomas.
In this SelectScience® interview, we speak with Dr. Richa Singhania, Director of the Starr Foundation Glionoid Translational Core at Weill Cornell Medicine, to learn about the key considerations in successfully recreating gliomas in a dish.
3D organoid co-cultures replicate glioblastomas in vitro
“We've successfully developed a cerebral organoid-based 3D model of human glioblastoma to replicate the fundamental biology of these tumors,” says Singhania. “We work towards developing a personalized, targeted treatment for brain cancer patients by integrating cerebral organoid technology with automated high-throughput screening.”
For modeling glioblastoma in organoids, the core facility uses a co-culture model. Patient-derived glioma stem cells are co-cultured with cerebral organoids to induce tumor formation. “We call our model GLICO – glioblastoma cerebral organoids – where these glioma tumors grow within the context of the human brain microenvironment as provided by the organoids,” explains Singhania.
GLICO generation is a multi-step process. First, glioma stem cells are isolated from fresh patient tumor biopsies. They are then tagged with a fluorescent reporter to enable visualization of tumor growth within the organoid in real time. Finally, these tumor cells are co-cultured with mature cerebral organoids that are generated from human embryonic stem cells (hESCs) or induced pluripotent stem cells (iPSCs).
“Within a couple of days after co-culturing, tumor cells start infiltrating the organoid,” notes Singhania. “Following this invasion, the tumor cells proliferate and migrate throughout the organoid, ultimately forming tumors that closely resemble the glioblastoma seen in patients.” These tumors mimic original gliomas in terms of their characteristic features, such as infiltrative behavior, as well as key genetic and molecular features.
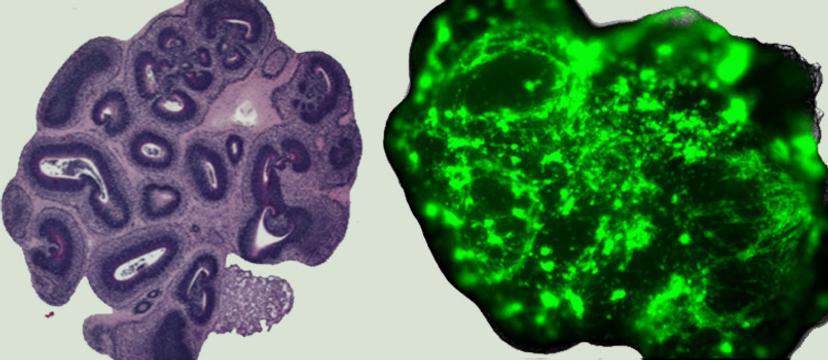
An image of the 'normal' and 'glioma' cerebral organoids Image © finelab.weill.cornell.edu
A human-specific brain microenvironment
GLICO tumors are able to closely recapitulate the phenotypic and molecular heterogeneity observed in patients' gliomas, a feat that traditional cell lines or animal-based models have repeatedly failed at.
“The first and foremost feature that we're able to capture in this GLICO model is how the tumors grow in the same manner as they would within the human brain,” notes Singhania. “This means, we can now study the behavior of the tumor and potentially find targets to stop its invasion into the brain.”
In this setting, the organoids serve as mini-brains, acting as a surrogate for the human brain. “We believe that the GLICO platform offers a unique tool to study fundamental tumor biology as it occurs in humans,” notes Singhania. “It enables us to explore tumor-host interactions in the context of a natural human tumor microenvironment and test potential drugs.”
Conventional animal models, where human cells are grown within the mouse brain, present a cross-species problem, making it impossible to capture the essential cellular crosstalk between the tumor cells and healthy cells. The organoids, on the other hand, are derived from human stem cells, making it possible to recreate a human-specific brain microenvironment. “The advantage of this model is that it can be readily manipulated, genetically and molecularly, for studying the functional consequences of gene mutations or alterations,” explains Singhania. “As such, it offers a robust platform for drug discovery and toxicology studies.”
Developing high-quality organoids: The right conditions
Due to the complexity of the human brain, cerebral organoid generation entails a four- to six-week-long process. “It is very critical to start with high-quality human pluripotent stem cells that have been cultured under the right conditions, to begin with,” Singhania says.
“Corning has a fantastic product line to support our research with brain organoids. We use the Corning® Matrigel® hESC-Qualified Matrix for culturing human pluripotent stem cells.” Extracellular matrices are an important consideration in generating 3D cellular models. They not only lend structural support for the cells to self-assemble but also provide biochemical signals for cell migration and growth.
Singhania continues: “We have a very critical step in the organoid production timeline where we have to embed the embryonic bodies into a Matrigel droplet. For this step, we utilize the Corning® Matrigel® Basement Membrane Matrix. Here, again, the composition of the Matrigel is important, and we work closely with Corning representatives to obtain good reproducibility. Both these products that we use in the pipeline play a key role in maintaining the health and the right differentiation of the organoids.”
Moreover, each lot of the Corning® Matrigel® Matrix is optimized to form a stable 3D dome structure that enables organoids to grow from both healthy and diseased cell origins. “We also use Corning's spheroid microplates, which we find extremely useful for our automated organoid-based drug screening platform,” Singhania adds.
The patients’ own mini-brains
An important project at the Weill Cornell Medicine brain organoid facility is to use this organoid-based glioblastoma model for performing clinically relevant drug screening. An automated high-throughput screening platform facilitates the screening of drug libraries in GLICO tumors.
“Going forward, we want to derive autologous brain organoids – our patients’ own mini-brains in a dish – to generate and utilize patient-specific tumor organoids as a precision medicine tool for tumor modeling and personalized drug testing,” explains Singhania. The goal of such an endeavor is to essentially predict if a patient with a particular mutation or a gene abnormality would benefit from a proposed treatment in the clinic.
“Pairing the patients' tumor cells with their own mini-brains would be pathbreaking. It will give us the opportunity to create personalized solutions for brain cancer patients,” Singhania adds.
The future of brain organoids in translational research
Brain organoids have catapulted neuroscience research in a way that was once inconceivable. A close-to-real human brain microenvironment is now feasible at a fraction of the cost, time, and resources required by traditional mouse models.
“There's been continuous interest and improvement in the organoid technology, which is causing a paradigm shift in the study of neurological disorders,” notes Singhania. “Recent organoid studies have focused on brain regional interconnectivity and neural circuit formation in a dish, providing new avenues to explore neuropsychiatric disorders.”
In light of the COVID-19 pandemic, brain organoids were used to model the viral infection and inspect its effects on the central nervous system. Singhania continues: “Research groups performed experiments where they infected the brain organoids with the novel coronavirus. They demonstrated that SARS-CoV-2 preferentially targeted the choroid plexus, breaking the brain-cerebrospinal fluid barrier, allowing leakage of immune cells and cytokines and triggering neuroinflammation.”
“Brain organoids hold a tremendous potential,” Singhania summarizes. “It’s an unprecedented opportunity for patient-centric research. With each passing day, the technology is only going to get better to make these model systems more predictive for patients.”
Do you use Corning Life Sciences products in your lab? Write a review today for your chance to win a $400 Amazon gift card>>