The role of syndromic testing in the era of COVID-19
27 Nov 2020

COVID-19 has changed our world. Since the onset of this pandemic, there have been millions of cases globally which have caused hundreds of thousands of deaths. Due to a lack of adequate testing and infection data from around the world, the cumulative infection and death rates from the COVID-19 pandemic most likely underestimate the pandemic’s true impact. To address these problems, researchers, health officials, and diagnostic companies around the world have come together to better understand the SARS-CoV-2 virus, protect communities from outbreaks, and produce robust diagnostic testing.
In this guest editorial, Dr. Christine Ginocchio, of BioFire Diagnostics, shares the importance of syndromic testing in this new age of viral infection and details the pathogenic trends throughout the different phases of the COVID-19 pandemic.
What is syndromic testing?
Out of the hundreds of SARS-CoV-2 diagnostic tests being developed and currently in use, syndromic testing is of particular significance as we look for ways to effectively combat the pandemic. Syndromic testing is the process of using one test to simultaneously target and detect multiple pathogens that cause overlapping signs and symptoms. Syndromic respiratory testing, such as the emergency use authorized BioFire® Respiratory 2.1 (RP2.1) Panel (EUA)*, detects SARS-CoV-2 as well as 21 other common respiratory viruses and bacteria responsible for illnesses such as COVID-19, the common cold, influenza, pneumonia, bronchiolitis, and even whooping cough. This comprehensive detection often influences patient management by tailoring therapy and limiting the unnecessary administration of antibiotics.
BioFire® Syndromic Trends
To illustrate the significance of syndromic testing in a pandemic setting, I’d like to introduce BioFire® Syndromic Trends (Trend). Trend is a software feature developed by BioFire to monitor infection rates throughout the world. This is done by gathering and aggregating de-identified pathogen data from BioFire® FilmArray® Systems housed in participating institutions. Trend provides pathogen circulation data that offers valuable insight into pathogen-specific seasonality trends over time. This data, which can be viewed at SyndromicTrends.com, has been crucial in understanding how the prevalence of other respiratory pathogens has been affected during the different phases of the COVID-19 pandemic.
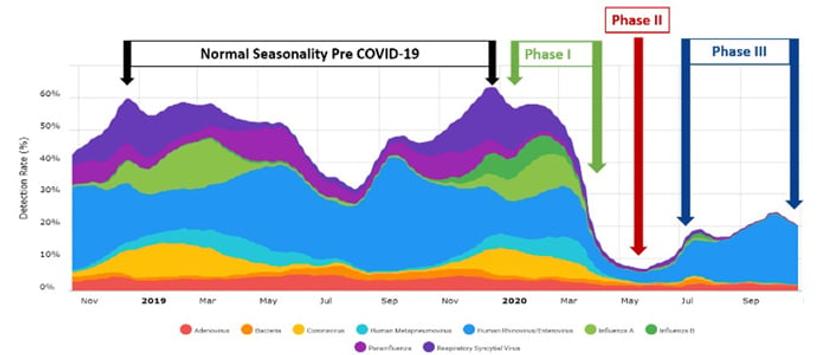
Phase 1 (see Figure 1) of the pandemic in the United States occurred between January and March of 2020. During this time of low prevalence of COVID-19 infection, common respiratory pathogens circulated, mirroring seasonality trends from years past. Total infection peaks for non-SARS-CoV-2 respiratory pathogens were reported to be between 50-60%, according to Syndromic Trends. As the 2020 US respiratory season was ending and we moved into spring and summer, we also moved into Phase 2 of the pandemic, which was characterized by a widespread acceleration in the prevalence of COVID-19 infection throughout the US. As the classical winter respiratory seasons end, positivity rates for total respiratory infections identified by the BioFire Respiratory Panels usually decrease to around 30-35%. Interestingly, during the pandemic, the infection rates for non-SARS-CoV-2 pathogens dropped to around 10% during Phase II, according to Syndromic Trends. Several factors such as quarantines, school and business closures, social distancing, and infection control precautions (e.g. masks and hand hygiene) likely contributed to this decline. If we prevent the spread of SARS-CoV-2 we are also not spreading other common viral respiratory pathogens. However, as the country started to reopen in Phase III, the cumulative positivity rates of the other respiratory pathogens increased to approximately 25% in September, which is roughly 20% lower than expected at this time of year.
Benefits of syndromic respiratory testing
During the early days of the pandemic, there was a lot of discussion about diagnostic testing and, more specifically, where syndromic testing would fit in. Initially, syndromic testing was used as a frontline test even though it did not include SARS-CoV-2, because no such test existed at the time. Eventually, targeted testing for SARS-CoV-2 became available. When targeted testing was used in conjunction with syndromic testing, both tests were extremely useful in ruling in other diseases and ruling out COVID-19, and vice versa. Now, with the release of the BioFire RP2.1 Panel (EUA)* with SARS-CoV-2, COVID-19 testing is part of syndromic respiratory testing, improving hospital efficiency by testing for the most common respiratory pathogens with one test, producing results in about 45 minutes.
Patients and healthcare workers alike have experienced the benefits of syndromic respiratory testing during the COVID-19 pandemic. For patients, it has helped rule in and out certain respiratory pathogens, including SARS-CoV-2. Some patients may be infected with SARS-CoV-2 and another respiratory pathogen, including treatable pathogens such as influenza and Mycoplasma pneumoniae1,2 – another reason why syndromic respiratory testing is so important.
This information has been key in allowing healthcare professionals to make informed decisions about personal protective equipment, isolation precautions, and quarantine plans, among other things. Syndromic respiratory testing has also benefited healthcare workers who are frequently exposed to COVID-19. Those that become symptomatic are able to gain valuable insight into what they are actually infected with. This has enabled hospitals to make informed decisions about employees returning to or continuing to work. COVID-19 testing will continue to be a front-line test in the outpatient setting. The BioFire RP2.1 Panel (EUA)* will be especially important for patients with specific risk factors that are more prone to severe respiratory infections. Furthermore, the BioFire RP2.1 Panel (EUA)* is an effective front-line test for healthcare workers when a rapid result is critical, such as when deciding on ICU admission, infection control, treatment, or in identifying co-infections.
Outside of the COVID-19 pandemic, the benefits of syndromic respiratory testing abound. Syndromic testing helps guide the use of antivirals such as oseltamivir and other antimicrobials, leading to improved antimicrobial stewardship through shorter courses of antibiotics and a reduction in overall use3-6. Syndromic respiratory testing has led to reduction in hospital length of stay3,6, and more appropriate, cost-effective use of infection control measures and ancillary services3-6. For high-risk populations that suffer from co-morbidities or are immunocompromised, syndromic testing is important in understanding the severity of infections as well as helping diagnose co-infections. Syndromic testing is also vital in transplant patients where the detection of viral pathogens, such as adenovirus, may be critical in tailoring care.
Looking ahead
As we move into subsequent phases of the pandemic and the economy begins to open up, common respiratory pathogens are starting to reemerge. Being able to detect whether an individual has COVID-19 or something else will be key in maintaining the progress that we have made during this pandemic. While the future course of the COVID-19 pandemic and its full impact are uncertain, we can be certain that syndromic respiratory testing will continue to provide rapid, comprehensive results that will arm healthcare workers with the information they need to make smart, effective decisions to continue the fight against respiratory illness.
Find out how to rapidly test for multiple disease-causing microorganisms with syndromic testing in this comprehensive article and learn more about the BioFire® Respiratory 2.1 (RP2.1) Panel (EUA)* here >>
*Emergency Use Authorization
*This test has not been FDA cleared or approved; This test has been authorized by FDA under an EUA for use by authorized laboratories; This test has been authorized only for the detection and differentiation of nucleic acid of SARS-CoV-2 from multiple respiratory viral and bacterial organisms; and this test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
Easom N et al. Influenza Other Respiratory Viruses. 2020;00:1–6
Kim D, et al. Rates of Co-infection Between SARS-CoV-2 and Other Respiratory Pathogens. JAMA. 2020;e206266.
Brendish NJ. www.thelancet.com/respiratory http: //dx.doi.org/10.1016/S2213-2600(17)30120-0
Brendish N et al. European Respiratory Journal 2018, 52 (2): 1800555; DOI: 10.1183/13993003. 00555-2018
Echavarría M. et al. Journal of Clinical Virology 108 (2018) 90–95
Duan S et al., Clinical Microbiology and Infection, https://doi.org/10.1016/j.cmi.2019.06.01