Understanding the link between MGUS and multiple myeloma: an expert view
Clinical consultant at Siemens Healthineers explores the link between MGUS and multiple myeloma and reveals how immunoglobulins can be used to effectively aid disease detection
14 Nov 2022

In this exclusive SelectScience® interview, we speak with Dr. John V. Mitsios, PhD, Senior Clinical Consultant at Siemens Healthineers, to find out more about monoclonal gammopathies of undetermined significance (MGUS) and its close link with multiple myeloma, a rare form of blood cancer characterized by excessive production of plasma cells found in the bone marrow. Mitsios also explains the value of investigating free light chains to effectively evaluate MGUS and multiple myeloma, and how these small proteins could serve as vital tools to better support clinicians by ensuring that accurate diagnostic information is gathered in a timely manner. Dr. Mitsios also explains how to best interpret these results to improve patient care and allow us to observe MGUS progression in a more impactful manner.
A strong link: MGUS and multiple myeloma
MGUS is a blood condition that occurs when plasma cells develop abnormally within the bone marrow, creating an elevated level of M protein, also referred to as M spikes, monoclonal proteins, or paraproteins. Unlike infection-fighting immunoglobulins (also referred to as antibodies), M proteins have no utility in the body. In some cases, they can saturate the bloodstream, depositing into tissues and potentially leading to organ dysfunction. Plasma cells are a type of immune cell that serve as vital components of the immune system. These white blood cells are found within many tissues associated with the immune system and develop from activated B cells, which originate in the lymphoid organs and secrete specific antibodies in response to the presence of cognate antigen. In MGUS, these plasma cells secrete higher levels of M proteins throughout the body. In the majority of cases, MGUS is asymptomatic and does not require any treatment, although some patients may experience peripheral neuropathy, which leads to burning in the hands and feet. In rare cases, MGUS can progress into multiple myeloma, and high levels of M proteins can serve as an indicator.
Multiple myeloma is a rare form of blood cancer, characterized by excessive production and improper function of plasma cells that can also lead to abnormal quantities of M protein, which can progress to affect the bones, kidneys, blood, and other tissues. Moreover, excessive levels of M protein can induce plasma cells to mass together to form tumors, particularly within bone marrow tissue.
To learn more about the link between MGUS and multiple myeloma, we spoke with Dr. Mitsios. “Multiple myeloma can damage the bones, immune system, kidneys, and red blood cell production. Often, clinical symptoms may not be present, or they may even be non-specific, such as loss of appetite, bone pain, and fever,” shares Mitsios. “To diagnose this condition, clinicians order a battery of tests, including a basic metabolic profile (BMP). However, if there is strong clinical suspicion for multiple myeloma, additional tests such as serum protein electrophoresis and serum free light chains are also ordered. The constellation of symptoms and laboratory findings will then lead to the diagnosis.” Thus, the diagnosis of multiple myeloma relies on a combination of both extensive laboratory and clinical findings.
Despite similarities between MGUS and multiple myeloma, there are significant differences. “Patients with MGUS can remain stable for many years. However, MGUS can progress to multiple myeloma or another blood disease, such as amyloidosis or Waldenström’s macroglobulinemia,” shares the clinical consultant. “However, this occurs in a small percentage of patients with MGUS. Most people with MGUS remain well for many years without ever developing active disease. As there is a slight risk of progressing to multiple myeloma, patients with MGUS should have their protein levels monitored through regular blood tests under the guidance of their doctor.”
How does evaluation of free light chains help us understand and assess multiple myeloma?
Antibodies are large, flexible immunoglobulins produced by B-lymphocytes, with approximately 1 trillion B-lymphocytes existing within the human immune system. Antibody classes are formally identified as isotypes. Many of these isotypes share structurally similar features, yet each holds unique properties that allow them to perform within many diverse biological roles. Immunoglobulin class is governed by numerous factors associated with antibody composition and the conformation of the heavy chains, as well as the number of constant domains and hinge regions. The physiochemical and serologic properties of the heavy chains differ among the isotype classes, and in essence, the heavy chains determine the biological properties of the antibody. The five classes of immunoglobulins are immunoglobulin G (IgG), immunoglobulin A (IgA), immunoglobulin M (IgM), immunoglobulin D (IgD), and immunoglobulin E (IgE).
To explore this further, Dr. Mitsios explains, “Plasma cells produce and secrete the antibodies that help fight off infection by recognizing and attaching to bacteria as well as viruses. If a virus or bacteria is identified by an antibody, it is targeted for removal by the immune system.” Mitsios continues, “You can think of an antibody like a ‘tag,’ where it notifies the immune system that there is something that needs to be removed. A typical antibody is composed of two immunoglobulin heavy chains and two light chains. The variation in heavy chain polypeptides allows each immunoglobulin class to function in a different type of immune response, or during a different stage of the body’s defense.”
Mitsios continues, “In addition, there are two different types of light chains, called kappa and lambda. Immunoglobulins are formed when light chains link up with these heavy chains. When the light chains link up with the heavy chains, they are known as bound light chains. However, plasma cells also produce a small amount of extra light chains that do not bind with heavy chains and are released into the bloodstream. These unlinked chains are known as free light chains (FLC). Patients with multiple myeloma will have an overproduction of the immunoglobulins and light chains.” In other words, the four chains of the antibody molecule consist of two identical heavy and light chains, which are linked together by noncovalent interactions and covalent disulfide bonds. The two identical light chains are classified as either kappa (κ) or lambda (λ), with each antibody owning only one of each, and not both configurations.
Diagnosis: multiple myeloma
The IMWG guidelines recommend free light chain testing for detecting multiple myeloma.
Dr. John V. Mitsios Senior Clinical Consultant, Siemens Healthineers
Regarding the diagnosis of multiple myeloma, Mitsios states, “Patients can present with non-specific symptoms, such as back pain, bone pain, or fatigue. However, these patients may have calcium elevation (C), renal dysfunction (R), anemia (A), or bone lesions (B), referred to as the CRAB criteria.” As well as being used to summarize these four common signs of multiple myeloma, the CRAB criteria can also be used to distinguish between active and symptomatic multiple myeloma and MGUS. Recently, updated diagnostic and staging criteria, known as SLIM CRAB criteria, have also been implemented. To explain this further, Mitsios says, “The SLIM CRAB criteria represent sixty percent (S) or greater clonal plasma cells in the bone marrow, plus a light chain (LI) ratio greater than or equal to 1001 (for the The Binding Site’s Freelite assays, but 702 for the Siemens Healthineers N Latex FLC assays), along with lesions greater than one found by an MRI scan (M).” In addition to these criteria, the M proteins are quantified by various techniques. “We can measure intact immunoglobulins, or we can measure free light chains. Additional tests such as serum protein electrophoresis and immunofixation are important in isotyping and quantifying the M protein spike. Of course, we can measure serum free light chains as well. Today there are many tools available to assess these patients,” explains the clinical expert. It is important to note that a slow, incorrect diagnosis could lead to treatment delays, ineffective treatments, and poor patient care. Therefore, it is vital to use all appropriate diagnostic tools to ensure optimum patient care is delivered.
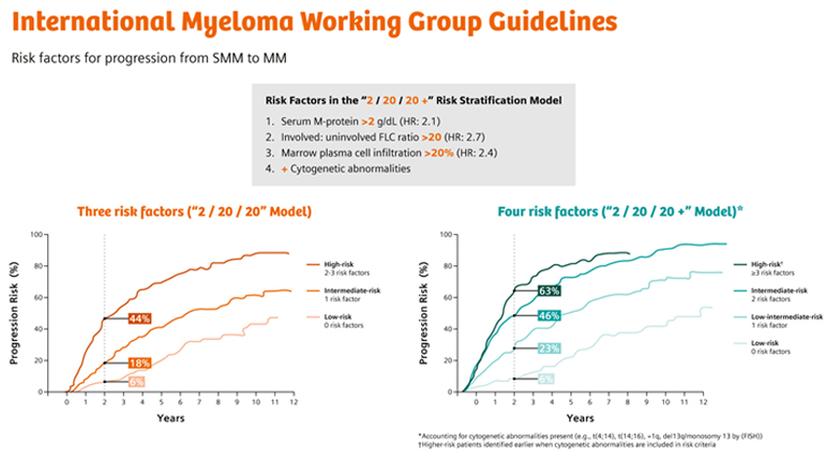
MGUS to multiple myeloma progression: seeing the clearer picture
Free light chains (FLC) are often used to monitor disease progression, marked by abnormal production of either the kappa or lambda light chains. By routinely monitoring FLC, clinicians can track the progression from MGUS to multiple myeloma.
When comparing a healthy person to a patient developing multiple myeloma, both the immunoglobulin complex (heavy chains and bound light chains) and the FLC are measured. In a healthy patient, FLC concentrations are usually very low. With multiple myeloma, the concentration of immunoglobulins may not be significantly elevated. However, FLC concentration can be notably increased, and this is clinically significant.
“In my opinion, measuring FLC allows one to have a better picture as to the overall status of the patient, especially in the context of MGUS, when there is a concern for progression to multiple myeloma,” shares Mitsios. Notably, the FLC are freely filtered by the kidneys, and “the smaller FLC kappa complexes are slightly more efficiently filtered by the kidneys compared to the large lambda molecules, resulting in a half-life time of 2–3 hours for kappa and 4–6 hours for lambda. The short half-life time is important for monitoring therapy response in monoclonal gammopathies, as the complete immunoglobulin complex shows a much longer half-life time, which is shortest for IgG at about 3 weeks. Like other small proteins (such as cystatin C and a1-microglobulin), FLC are reabsorbed from urine by renal tubules.”
Now that we understand the significance of evaluating FLC to observe MGUS progression, how do we interpret the results? Mitsios states, “We should always interpret any type of FLC data together with the whole clinical picture. We look at all the data available from the laboratory (for example, calcium, creatinine, hemoglobin, and serum protein electrophoresis), along with imaging results and the clinical presentation of the patient, to interpret FLC data appropriately.” To help interpret the results even further, the free kappa to free lambda ratio is also explored. For instance, a high free kappa to free lambda ratio strongly indicates that the involved chain is the kappa. Alternatively, a low kappa-to-lambda ratio indicates that the FLC is lambda. “Nevertheless, I always prefer to look at both the involved and uninvolved FLC. The rationale for this is that these findings can be skewed depending on certain clinical conditions. For example, studies have shown that patients with chronic kidney disease may form higher-order structures of FLC, such as dimers and trimers. Specifically, kappa chains are more susceptible to these higher structures. Therefore, in patients with chronic kidney disease, there may be an increase in these higher-ordered structures.” For this reason, it is important to note that an assay that preferably detects dimers and trimers may result in false elevation of the kappa chains, while the lambda chains remain the same. This can result in an elevated and inaccurate ratio. “This may result in the clinician incorrectly interpreting the presence of myeloma and lead to potentially unnecessary medical examinations. I personally like to follow a stepwise approach when looking at FLC data. This includes: 1) Explore renal function (e.g., creatinine or cystatin C), 2) explore the involved FLC, 3) explore the uninvolved FLC, and 4) observe the ratio,” said Mitsios.
“What's important about these monoclonal gammopathies is how we properly assess the patients. In a paper published by Katzmann et al., a study looked at different screening algorithms associated with monoclonal gammopathies. Despite being published in 2009, this study really does highlight the importance of adequate and appropriate screening processes in the laboratory. For example, using serum protein electrophoresis (SPEP), immunofixation (IFE), and FLC quantification resulted in a high degree of diagnostic accuracy in patients with multiple myeloma and/or MGUS (>90%).”
Free light chains: new diagnostic possibilities
Interestingly, there are other conditions for which FLC measurements can be used to benefit and further improve patient evaluation. “FLC may play an important role in evaluating autoimmune diseases, such as multiple sclerosis, and further studies are being conducted to address this very question,” Mitsios concludes. Findings so far suggest that evaluation of FLC can be considered as a reliable method for diagnosis and monitoring of several disorders, particularly in cases of plasma cell disorders. FLC therefore play an important role in the prognosis of patients with MGUS in addition to being key diagnostic markers for multiple myeloma. The findings highlight real value for evaluating FLC and disease progression, which could have significant clinical impact for patients who may or may not go on to develop multiple myeloma.
References
Rajkumar SV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014 Nov;15(12):e538-48. https://pubmed.ncbi.nlm.nih.gov/25439696/
Henriot B, et al. Prognostic value of involved/uninvolved free light chain ratio determined by Freelite and N Latex FLC assays for identification of high-risk smoldering myeloma patients. Clin Chem Lab Med. 2019 Apr 11;57(9):1397-1405. Available from: https://doi.org/10.1515/cclm-2018-1369
Learn more:
International Myeloma Working Group (IMWG) criteria for the diagnosis of multiple myeloma: Read the guidelines.
Evaluating monoclonal gammopathies of unknown significance (MGUS) with free light chain assays in clinical practice: View the webinar and read the white paper.
Add consistency to monoclonal gammopathy testing: Read the white paper.
Free light chain testing: Read the review of the guidelines and literature list.
FLC ratio in progression of smoldering myeloma: Read the white paper.
Understanding how FLC testing in patients with renal impairment can be misinterpreted as multiple myeloma: Watch the video.



