News & Articles
Selected Filters:
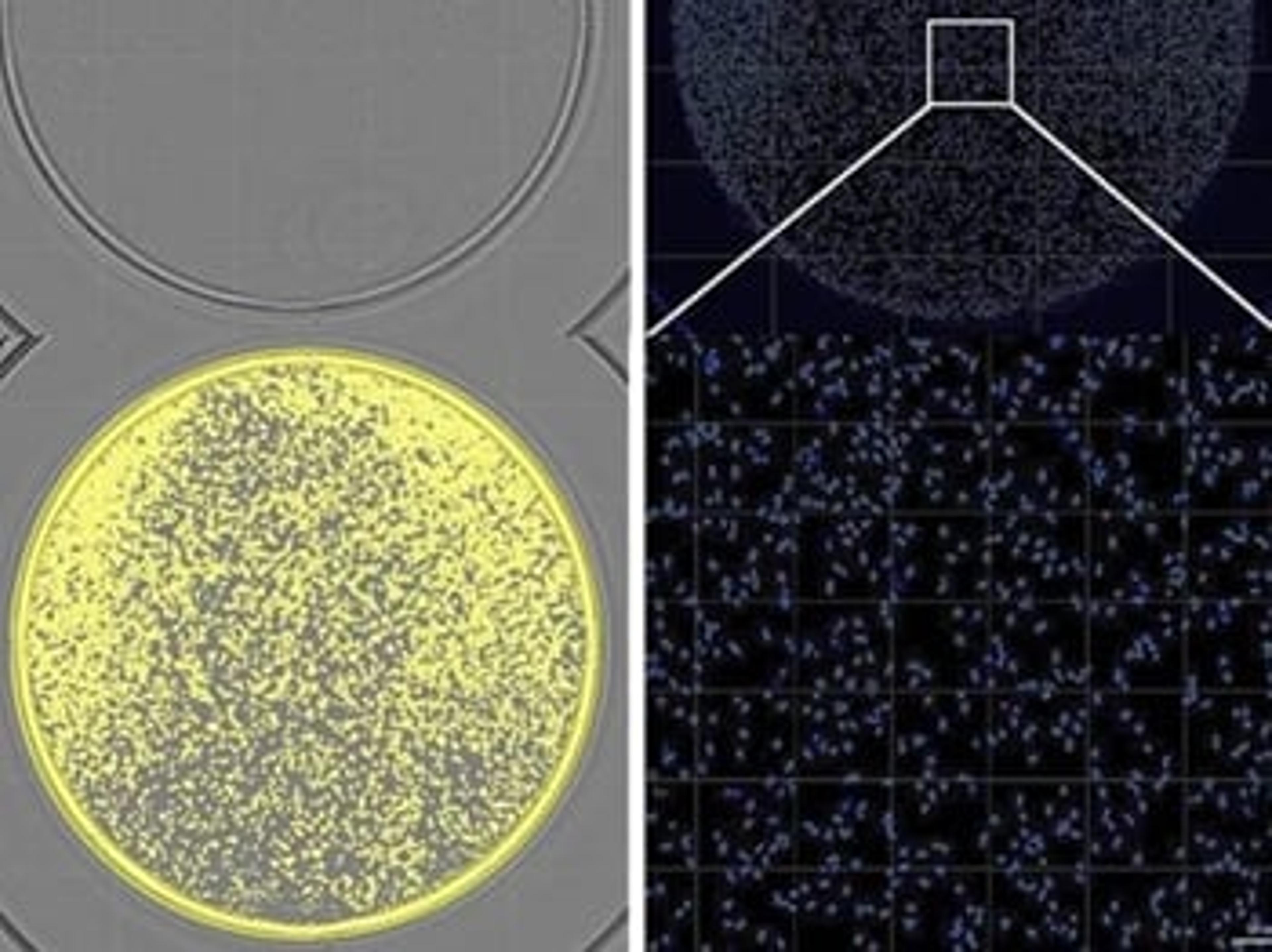
Menarini Silicon Biosystems launches the MSBiosuite Bioinformatic Platform
Cloud-based solution automates NGS data analysis for liquid biopsy and FFPE workflows
Nominations are open! Nominate your favorite new lab product of 2019 for a Scientists’ Choice Award
Tell us which new products made the most difference to your lab work and you’ll have the chance to win one of four $500 Amazon gift cards
Join SelectScience at Lab Innovations 2019 to celebrate the lab technologies scientists love the most & pick up your free T-shirt
Following last year’s success, we’re hosting our second annual event dedicated to celebrating our Seals of Quality program
Developing custom lentiviruses and enhancing transduction for more effective gene therapy
Find out how one lab is streamlining the development of lentiviruses and transduction enhancers to treat a range of genetic diseases
Red wine benefits linked to better gut health
Next-Generation Immunotherapy: Combining Virus-Specific T Cells with Other Immuno-Oncology Technologies to Treat Solid Tumors
Dr. John Connolly, CSO of Tessa Therapeutics, reveals how a new approach to cell-based therapy in cancer could circumvent severe immunotherapy side effects and effectively target aggressive solid tumors
FDA approves new drug for treatment-resistant forms of tuberculosis
Approval marks the second drug approved under the Limited Population Pathway for Antibacterial and Antifungal Drugs and signals FDA’s continued focus on facilitating development of new treatments to fight antimicrobial resistant infections
Discover Revolutionary Sample Management Technology at AACC 2019
The Atellica Solution from Siemens Healthineers promises more control and increased simplicity in your lab workflows
Nosopharm and Evotec Enter into Collaboration to Accelerate Development of Novel Antibiotics
New partnership to advance Nosopharm’s lead candidate to clinical stage
SMARTvector Technology Deployed in Successful IND Filing for Next-Generation CAR-T Cell Therapy
Phase 1 trial scheduled for early 2020 will be first CAR-T cell therapy clinical trial to employ SMARTvector technology
Avacta and Selexis Partner to Develop Cell Line for Clinical Manufacturing
Avacta’s first Affimer clinical candidate is a potent PD-L1 antagonist under development for solid tumor indications as the basis of bispecific and combination therapies
Personalized Adoptive T Cell Therapy: A New, Rational Approach to Immunotherapy
A small team of biotech scientists is targeting patient specific ‘neoantigens’ to improve response to adoptive T cell therapy in acute myeloid leukemia (AML)
Mogrify Awarded $555,000 USD Innovate UK Funding for Novel Bioinformatic Platform
Project to focus on data-driven cell conversions to produce cell therapies with potential application in wound healing, and oncology immunotherapy
New Treatment Has Potential to Minimize Damage After Heart Attack
The drug works by targeting the protein responsible for the death of heart cells during an attack
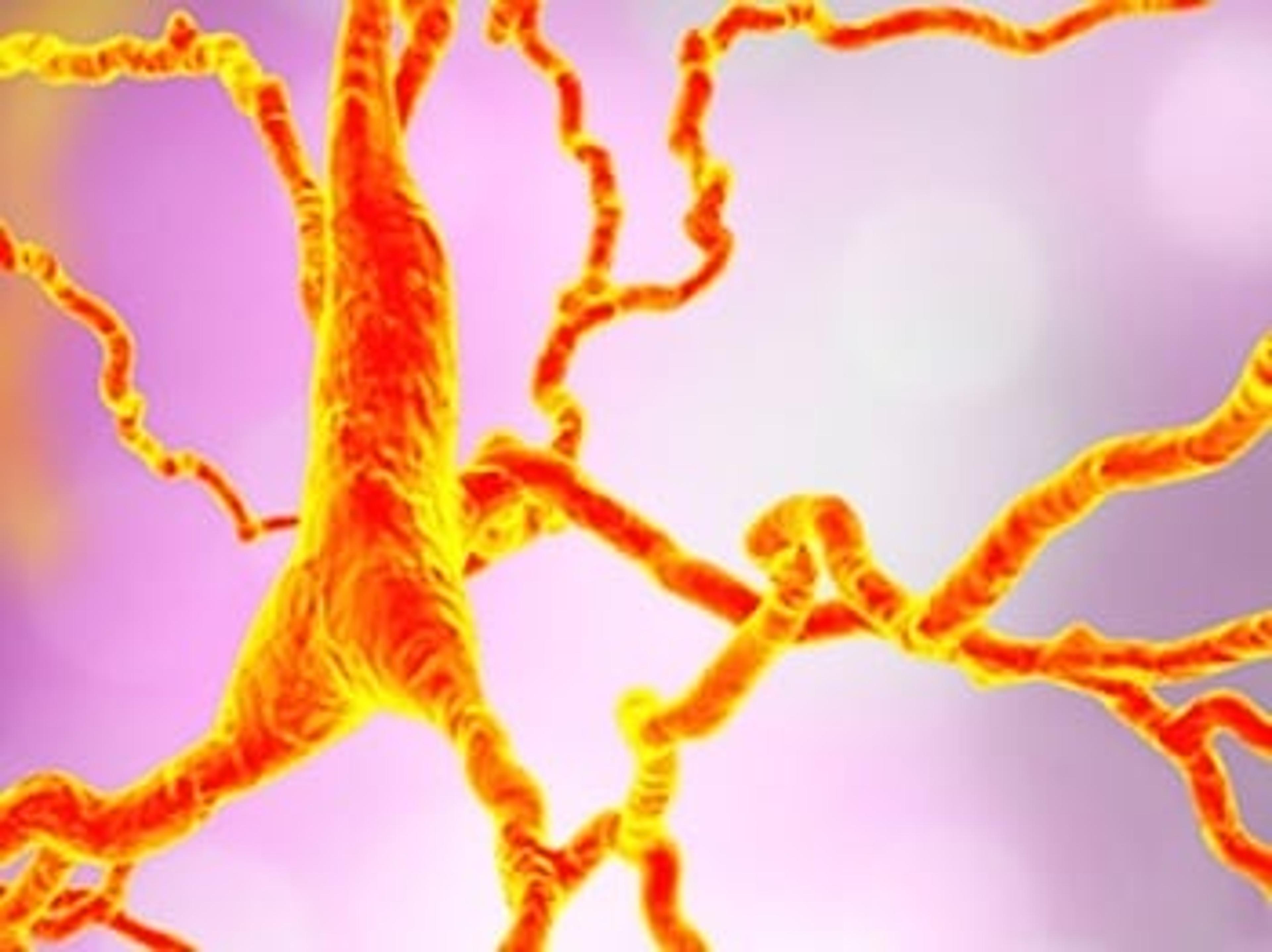
This Novel, Non-Amyloid Approach to Alzheimer’s Therapy Could Vastly Improve Outcomes
Immunology-focused pharma company INmune Bio is set to start first-in-man clinical trials with a promising anti-TNF compound that could revolutionize the treatment of Alzheimer’s disease and related symptoms
Researchers Use AI to Detect Early Signs of Alzheimer’s
Scientists at the University of Southern California have identified blood-based biomarker patterns that indicate early-stage disease