News & Articles
Selected Filters:
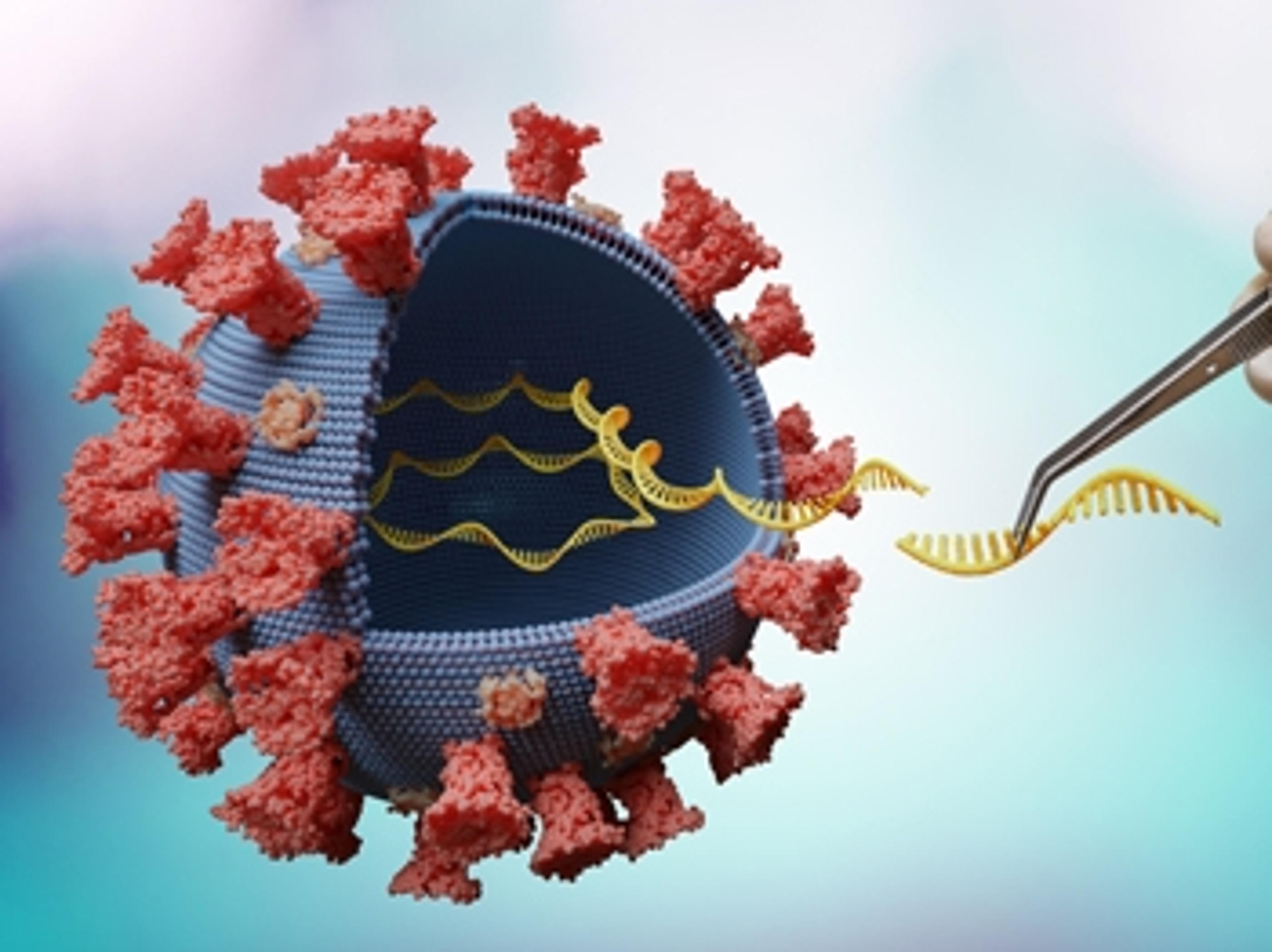
Lab-based coronavirus test aims to deliver up to 46,000 results a day
The testing capability of Mologic's kits is expected to relieve immediate testing pressures in the UK and Africa
All you need to know about HPLC columns in under an hour
Don't miss upcoming masterclass on how to maximize the lifetime and performance of your LC columns
Sensitive compositional analysis at the micro- and nanoscale, a secondary ion mass spectroscopy workshop
Watch this webinar to gain insight into SIMS technology and discover an innovative solution offering the current world record in SIMS lateral resolution
How to design high-parameter experiments to achieve more information from your spectral flow cytometry
Want a deeper insight from your spectral flow cytometry experiments? Our on-demand webinar answers all your key questions
How to design, optimize, and validate multiplex immunofluorescence panels
Hear from AstraZeneca expert Dr. Michael Surace on the biological and technical considerations for multiplex panels in this on-demand webinar
Roche develops new serology test to detect COVID-19 antibodies
The detection of these antibodies could help indicate if a person has gained immunity against the virus and inform treatment decisions
Temperature control solutions to support researchers in fight against COVID-19
Huber's high precision temperature control technology directed towards manufacturing test chemicals and PPE
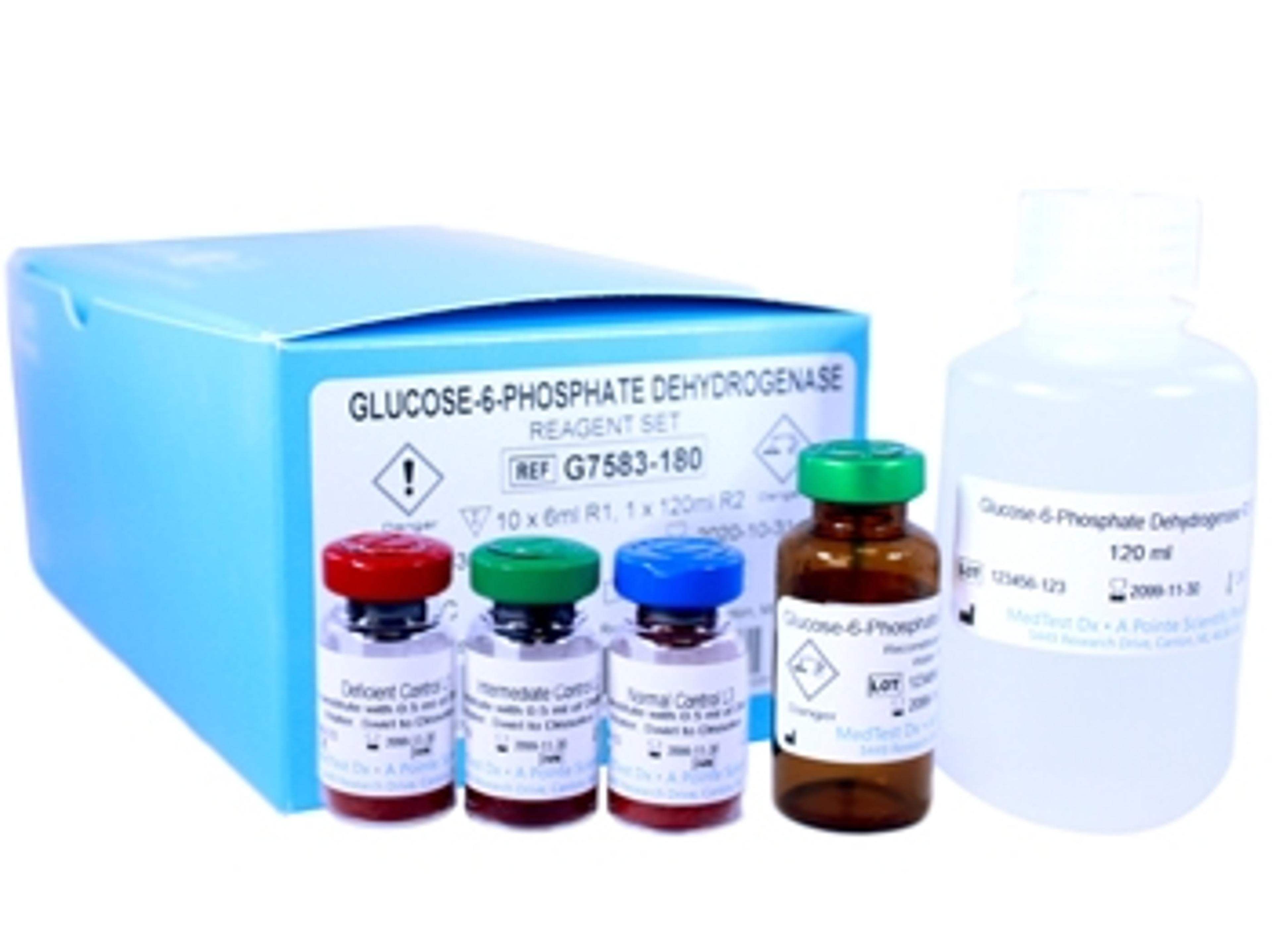
MedTest Dx manufactures quantitative test for G6PD with COVID-19 applications
The only FDA-approved test that measures G6PD provides key insight to inform drug therapy options
Bio-Rad to launch blood-based immunoassay kit to detect COVID-19 antibodies
The serology immunoassay kit aims to help clinicians diagnose the coronavirus by analyzing the immune response against SARS-CoV-2
From gene to genome: NGS workflow automation for homogeneity in sequencing
The head of molecular genetics at Munich Leukemia Lab highlights the benefits of sequencing automation to improve the success of leukemia therapy
High-throughput antibody discovery targeting cell surface receptors for signaling blocking in cancer
Join this upcoming webinar for a review of an antibody discovery campaign targeting a cell surface receptor implicated in cancer
FDA approves first saliva-based coronavirus test
Emergency use authorization granted for new biomaterial collection approach
Bruker and ANPC partner to support major new frontline response to combat COVID-19 threat
The strategic partnership with Australian National Phenome Centre (ANPC) aims to support the work of their researchers
Horizon Discovery offers CHOSOURCE platform for COVID-19-related therapeutics and diagnostics
Special licensing terms enable rapid access to CHOSOURCE platform to streamline SARS-CoV-2 therapeutic development and production processes
Next-generation bispecific antibodies clear circulating antigens while also targeting tumors
SelectScience hears from Prof. Harald Kolmar, TU Darmstadt, on an exciting new approach in cancer therapy
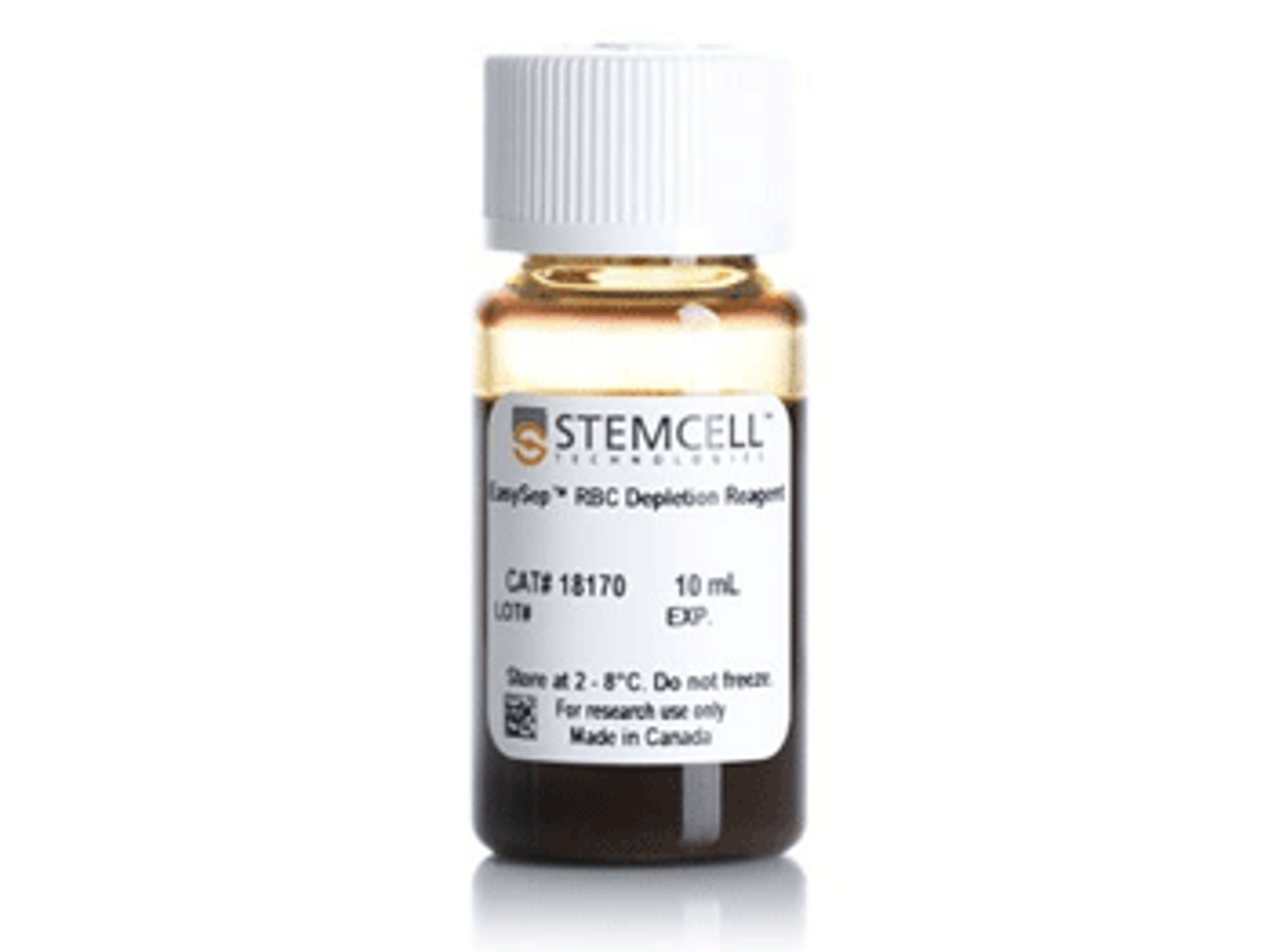
STEMCELL Technologies announces novel method for blood sample preparation for COVID-19 vaccine development
Curiox Biosystems and STEMCELL Technologies have announced a new method to safely prepare COVID-19 blood samples for vaccine research
Vote for Best New Life Sciences Product of 2019 in the Scientists’ Choice Awards
To be in with a chance of winning a $500 Amazon voucher, simply cast your vote for the product that has made the most difference to your research
SelectScience co-founder named top tech leader
Recognition comes as the publisher launches a series of Virtual Summits to support the science industry through the COVID-19 crisis
STEMCELL Technologies helps sequence COVID-19
Canadian biotechnology company STEMCELL Technologies has provided critical support to sequence the coronavirus, accelerating vaccine development
DeNovix launches new RNA quantification assay
DeNovix Inc. expands its fluorescence quantification range with the introduction of the new DeNovix RNA Assay.