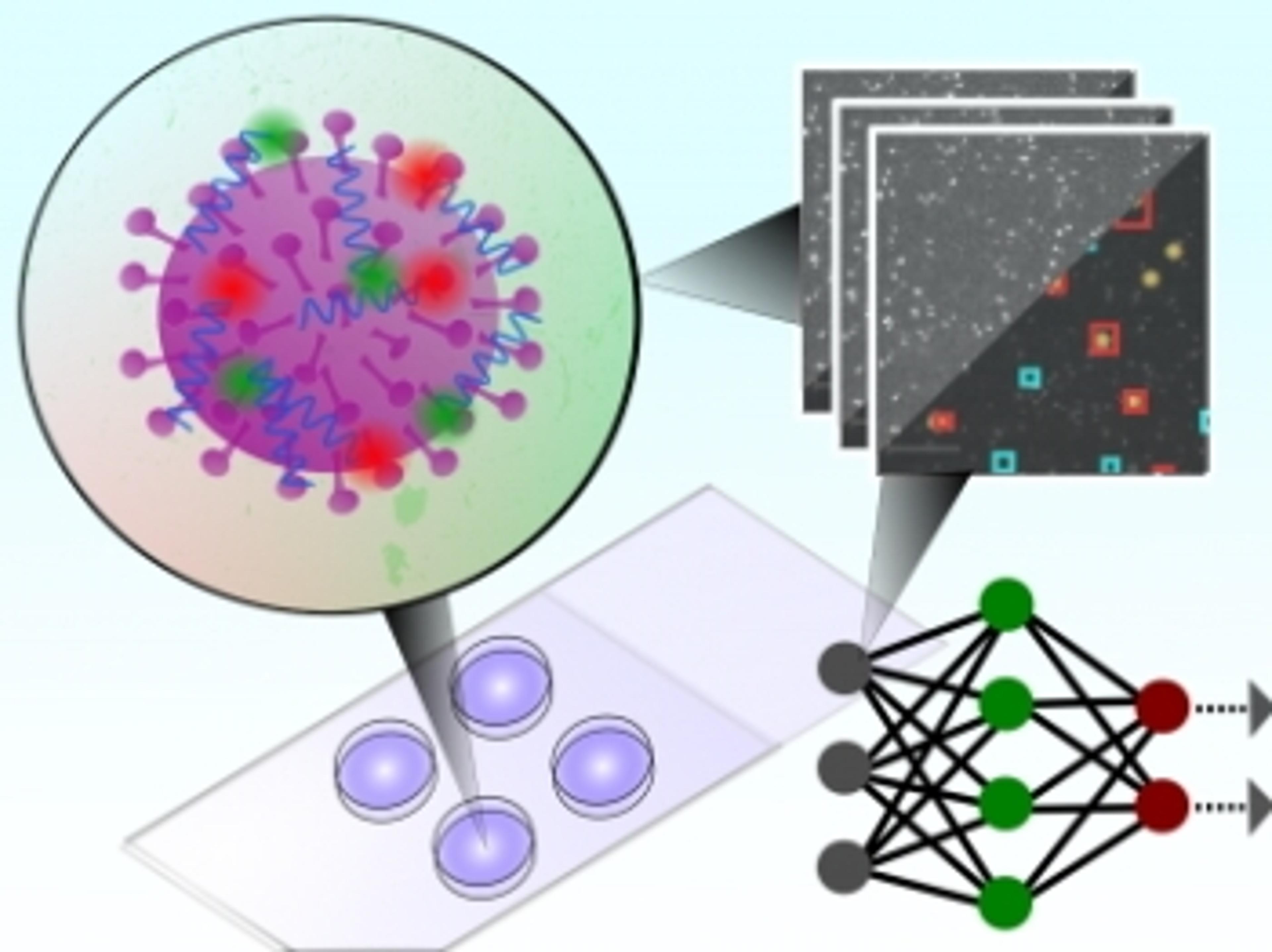
Study using Mission Bio’s Tapestri platform reveals clonal diversity underlying AML and therapy resistance
The study leverages the Tapestri Platform to illustrate the single-cell atlas of acute myeloid leukemia (AML) with multi-omics, describing the clonality underlying therapeutic resistance