Identifying tumor-specific cancer treatments using patient-derived tumor organoids
Dr. Benjamin Hopkins reveals how combining transcriptomics with organoid-based drug screening could bring effective therapies to cancer patients sooner
7 Mar 2022

Advances in sequencing technology have given rise to a surge of precision medicine approaches that use a patient’s genetic profile to tailor treatment strategies against disease. In the field of oncology, researchers working towards this goal are now able to identify the mutations driving tumor development and progression on an individual tumor level. However, the capacity to pair this information with effective therapies has remained limited, particularly for rare cancers, due to a lack of clinically relevant models.
In this article, we speak with Dr. Benjamin Hopkins, assistant professor at the Icahn School of Medicine at Mount Sinai, New York, to learn how his team is pairing comprehensive genomics with drug screening of high-fidelity patient-derived organoids to help identify effective therapeutic strategies for cancer patients.
A two-pronged approach to precision oncology
“My group focuses on asking two primary questions: what is the best drug for any given cancer patient, and what is the best patient population for emerging therapeutics?” begins Hopkins. “To answer both of these, we use three-dimensional tumor organoid models.”
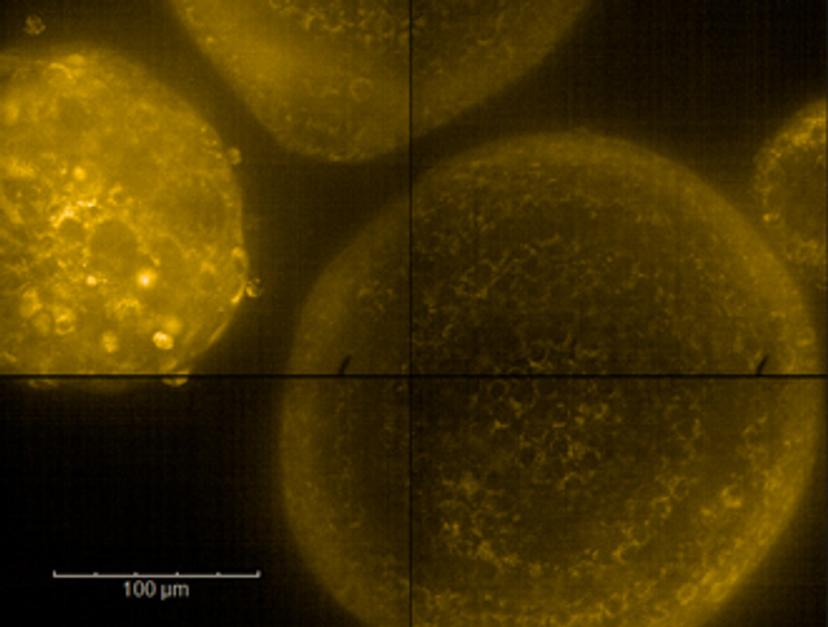
These 3D models, which act as ex vivo replicas of living cancer tissue, are developed in the lab using tumor samples from patients at Mount Sinai Hospital. Through combining transcriptomics with an automated high-throughput screening platform, collectively termed the Functional Genomics Pipeline, Hopkins’ team then works to identify the molecular mechanisms that lead to tumor-specific drug sensitivities.
He explains: “Using high-content imaging, we observe how the tumor organoids grow in normal conditions and how different drugs change their behavior over time, such as changes to growth or increased cell death. For drugs that we identify as sensitizing agents, we can then use RNA sequencing to ask what changes the drug is inducing in the tumor, along with combination drug screens to identify agents that target these specific pathways.”
A primary goal of this approach is to help identify cooperative agents with tumor-specific effects, which could then be leveraged to improve patient outcomes. “There are a lot of drugs that seem to have a good clinical role that don't necessarily kill the tumor cells,” explains Hopkins. “By combining functional observations with genomics, we’re able to learn what the first drug is changing, and then identify a second complementary drug that is targeting the pathway the tumor is using to evade the first treatment.”
In addition to identifying effective drug combinations, his team is also performing inverse screening to determine which patients are most likely to elicit the desired response to existing and novel therapeutics. “Here, instead of screening against the library of drugs, we take one drug and screen against the library patients,” explains Hopkins, adding: “For both of these screening modalities, we have four major tumor types of interest; non-small cell lung cancer, muscle-invasive bladder cancer, metastatic kidney cancer, and breast cancer.”
Developing high-fidelity tumor organoids: Tools for success
As with any research involving human samples, ensuring that experiments are consistent and reproducible is essential to generating clinically relevant data. For Hopkins’ work, selecting the appropriate culture vessels, matrix, and reagents to develop batches of organoid models is therefore vital. The organoid models his team develop are grown using Corning® Matrigel® matrix and housed within glass-bottomed Corning® 96-well sterile microplates, designed specifically for high-content imaging.
“One of the nice things about Corning Matrigel matrix is it’s really consistent,” he shares. “As long as you know what your protein content is, you can expect very similar results batch to batch, which is crucial for generating consistent and reproducible organoid findings.”
Once the organoids are developed, his team performs a number of fidelity checks, including paired genomic and pathologic comparisons, to ensure that the models accurately represent the physiology of the original patient tumor. Whilst recognizing alternative strategies for high-fidelity organoid generation, including the use of decellularized matrix, Hopkins notes a key advantage of Matrigel matrix-based culture is its ability to be scaled to high throughput. “Being able to reproducibly generate tumors is what allows us to take up to 480,000 images a week for a given tumor in our pipeline,” he says. “We can effectively remove the matrix as a variable in our analysis – and this is incredibly helpful given the level of variance inherent to 3D organoids.”
Why 3D?
The ability of 3D cell culture to recapitulate in vivo conditions more accurately than traditional 2-dimensional models has led to its wide adoption in the last decade. For Hopkins’ research, the benefits of this approach are clear. “In 3D, we’re able to interrogate with much higher fidelity how tumors will respond to a given therapeutic, and how the matrix, extracellular environment, or other cell types may contribute to therapeutic response,” he explains. “2D models often fail to capture the reciprocal relationship between a cell and its matrix, meaning you can miss out on some of the important biology that mediates drug sensitivity.”
Future outlooks
As the field of precision oncology gains increasing momentum, Hopkins sees organoids playing a significant role in active clinical assessment, with the ultimate goal of identifying curative care. His lab is already performing so-called rapid screens, in order to analyze the relative sensitivity of tumors to a reduced library of available standard of care agents. “Pancreatic cancer, for example, has two dominant standard of care therapies, but we currently don’t have a good way to identify which patients should get what,” he explains. “Using an organoid approach could provide a relatively quick turn-around for determining a patient’s sensitivity to multiple therapeutic options, as well as how that relates to the clinical responses seen in other patients.”
Longer-term, Hopkins suggests the combination of organoid models with transcriptomics data could also provide valuable insights for clinical trials. “Instead of leaving people on drugs until they fail, these types of datasets could enable clinical trials to be integrated with biomarkers of the desired response,” he explains. “Patients unlikely to respond could therefore be identified earlier, stratified, and subsequently moved onto a secondary trial.” He concludes: “Ultimately, this could provide a mechanism whereby more therapeutics could be tested, and trials could become better targeted for specific tumor subsets, to bring effective therapies to patients sooner.”
Do you use Corning Life Sciences products in your lab? Write a review today for your chance to win a $400 Amazon gift card>>