Next-generation bispecific antibodies clear circulating antigens while also targeting tumors
SelectScience hears from Prof. Harald Kolmar, TU Darmstadt, on an exciting new approach in cancer therapy
13 Apr 2020

Antibody therapeutics hold great promise for the treatment of cancer. As scientists strive to develop the most efficacious therapies, antibody engineering has become a key focus and provides vast possibilities to target specific cell types, as well as other molecules present in the body, for the treatment of disease. In this article, SelectScience hears from Prof. Harald Kolmar, professor of biochemistry at Technische Universität Darmstadt, about his work in antibody engineering and the development of a new bispecific antibody that can target solid tumors while also depleting tumor antigens present in plasma.
Tell us about the overall goals of your work on antibody engineering
We are currently engineering antibodies for various applications. For obtaining humanized antibodies from immunized animals, we use chickens as antibody producers. Target-binding antibodies are isolated by high-throughput FACS after gene transfer to yeast cells and ranked according to their affinities using the FortéBio Octet system, followed by humanization using a combined NGS (next-generation sequencing) and library screening approach. A set of final candidates is then validated by Octet analysis. A second field is related to pH-responsive antibodies. Our third route of antibody engineering comes into play in the field of personalized medicine. Here, we generate so-called anti-idiotypic antibodies that specifically recognize the B-cell receptor residing on the surface of patient lymphoma cells.
Please explain a little about your research into pH-responsive bispecific antibodies for cancer therapy
pH-responsive antibodies have different affinities at slightly different environmental pH values. This special type of antibodies is particularly interesting for cancer research since in the tumor tissue acidic conditions prevail. Hence, antibodies that display strong target binding at acidic tumor pH but are less affine at neutral pH in the blood and other tissues would be of value to avoid off-target effects. Classical examples for anti-tumor antibodies that would benefit from pH-responsive binding are antibodies targeting EGFR. This receptor is overexpressed in many cancers of epithelial origin and also exists at high density in other tissues, e.g. the skin, and a well-known side effect of therapeutic antibodies lacking pH-responsive binding is skin rash.
For other applications in cancer therapy, the opposite effect — namely reduced target binding at acidic pH — might also be beneficial. This is, for example, the case for CEACAM5, which is overexpressed in many cancers of the gastrointestinal tract. The extracellular domain of this receptor is frequently cleaved off by proteases and found in the bloodstream. Shed CEACAM5 supports the generation of tumor metastases and a strategy to reduce that risk is aimed at removing the shed CEACAM5 from the bloodstream. It has been shown that pH-responsive antibodies that release their target at acidic pH can be useful for blood clearance of the bound antigen.
Antibodies permanently undergo the process of endosomal recycling – they are taken up by blood endothelial cells, are transiently located in endosomes and transferred back to the cell surface and the bloodstream. Due to the acidic pH in the endosome, a pH-responsive antibody would release its bound target such as, e.g., CEACAM5, which is eventually degraded by the cellular lysosomal degradation machinery, while the target-free antibody is brought back to the bloodstream resulting in an efficient antigen removal process.
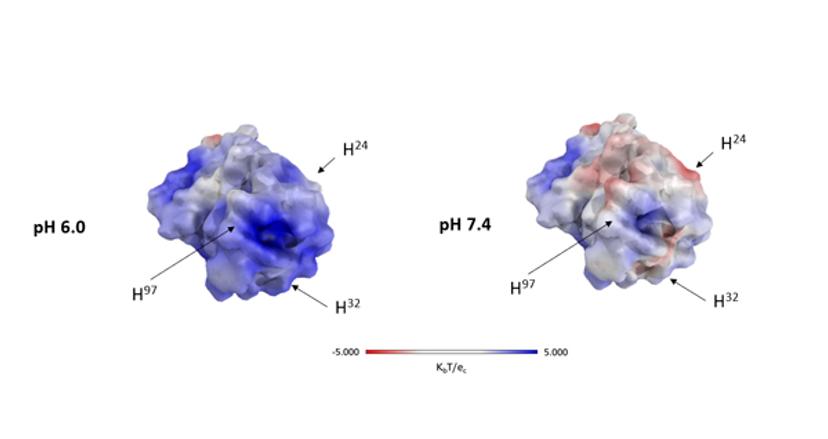
We constructed a model bispecific antibody, where one arm binds the target CEACAM5 in a pH-dependent manner and therefore can remove shed CEACAM5, while the other arm is not pH dependent and can bind the antigen on tumor cells, also in the acidic tumor microenvironment.
The particular advantage of bispecific pH-responsive antibodies lies in the fact that different functionalities can be programmed into one molecule thereby fine-tuning target binding in different pH environments. Likewise, one might be able to generate bispecifics that recruit immune cells with one arm while they bind their target preferably in the acidic environment of the tumor.
How are you using the FortéBio Octet system to help you carry out this work?
The FortéBio Octet was instrumental for screening panels of pH-responsive antibodies. First, we generated thousands of variants that were pre-screened by FACS and later required detailed testing for pH-dependent target binding or release. It is very easy to perform pH-dependent binding assays using the Octet since you can use biosensors with immobilized antibody candidates and then interrogate, in parallel, target binding and release at different pH values. Kinetic analysis directly provides information about the pH-dependent k-on and k-off values.
Which features of this technology help you to achieve your goals?
One very beneficial feature is that you can immobilize expressed antibodies directly from the cell culture supernatant to the biosensor without the need for purification. Hence, a medium-throughput screen to find candidates with desired target binding characteristics can be performed. Promising candidates can then be purified and used for further in-depth characterization using pure material at defined concentrations.
Finally, what is the future for antibody engineering and its application in cancer therapy?
From my personal perspective, I do not believe that a single strategy will be the solution. In my opinion, several strategies – most of them involve antibody engineering – may become even more important for the treatment of cancer than they are already today. First, antibody-drug conjugates, where drugs can be highly potent toxins, photoactivatable molecules that generate toxic radicals upon activation, or radioligands are promising tools to expand the therapeutic window in tumor therapy. Second, bispecific and multispecific antibodies that simultaneously address tumor cells and recruit cells of the immune system such as T cells or NK cells hold great promise for the future. Third, vaccination strategies that rely on stimulation of the patient’s immune system by confronting it with tumor-specific and patient-specific epitopes and, fourth, cell therapies that rely on modified immune cells that trigger an immune response against tumors provide additional important cancer treatment options.
Do you use the FortéBio Octet system in your lab? Write a review today for your chance to win a $400 Amazon gift card>>

