Transform your pharmaceutical workflow with cutting-edge chromatography data management
Find out how to meet tough quality control and assurance regulations with the latest resources, new methods, and exclusive interviews
16 May 2021

Quality control (QC) and quality assurance (QA) are paramount within the pharmaceutical industry, as well as in the development of beauty, nutrition, and homecare products. Products must be developed and manufactured to meet highly regulated standards, such as Food and Drug Administration (FDA) and EU Good Manufacturing Practice (GMP) regulations. In this compilation of new resources, we hear from scientists around the world about how the Waters Empower™ Chromatography Data System streamlines QC and QA workflows.

The cloud chromatography data system driving global operational efficiency at Amway
Find out how Caryn Weiss, senior research scientist at Amway, helped coordinate both QA and QC workflows across multiple testing sites by harnessing the latest cloud-based technology for remote chromatography data management.
Streamlining chromatography and data management for FDA approval
Discover why Dr. Anna Razynska, Director of Analytical Sciences, and Dr. Satya Ganti, Vice President of R&D at WES Pharma, chose to implement Waters Empower™ Software to help improve generic pharmaceutical productivity.

Achieving compliance in pharmaceutical manufacturing with cutting-edge data management
Read how pharma companies SM Biomed and Biolab are harnessing the latest chromatography data management tools for quality control and compliance to meet global regulatory standards.
More expert interviews on pharma and quality control:
- A simple step to upgrade your routine HPLC analysis: The Arc HPLC system aims to meet all the requirements of a top-of-the-line system in a cost-competitive package that fits into your LC routine with ease. Read article>>
- Leveraging technology in quality control: The power of partnership: Marcelo Shigueru Kamei shares how the latest chromatography solutions help his team at Cristália laboratory stay a step ahead as they manufacture quality pharma products. Read article>>
- Ensuring data quality and addressing chromatographic challenges in routine pharmaceutical analysis: Tools for success: Explore current trends in regulatory observations referencing chromatography analyses.Read article>>
- The use of Analytical Quality by Design workflows in the pharmaceutical industry: Discover a novel approach for in silico method development and robustness assessment.Read article>>
- New analytical methods for impurity detection: Learn how risk-based approaches can be implemented to improve the control of impurities. Read article>>
Other resources for chromatography data management:
- The making of a modern biopharmaceutical: Waters Corporation discusses the future trends in the biopharmaceutical industry including multi-attribute monitoring (MAM), biophysical analysis as a complement to analytical characterization, and the rise of mass detection as a foundational technology. Download here>>
- Ensuring food ingredient quality and consistency with a novel mass detection technology: Waters presents how this integrated system was comprised of the following technologies: ACQUITY UPLC H-Class System, ACQUITY QDa Mass Detector, and the Empower 3 Chromatography Software. Download method>>
- Analytical procedure lifecycle management drives method development at innovative CDMO: learn how Waters ACQUITY UPLC with PDA/QDa Detectors and Empower Software are integral to the early adoption of method lifecycle management at Hovione.Download method>>
- Monitoring Synthetic Peptide Impurities with Improved Confidence in Analysis: Waters demonstrates how mass detection can be readily incorporated into an existing optical-based workflow for the analysis of synthetic peptide impurities. Download method>>
- Waters Application Notes: Food Testing: Introducing the Xevo TQ-XS: Here, you will find a range of application notes, produced by Waters, covering the areas of food quality control, research, and safety.Download method>>
Your recommendations
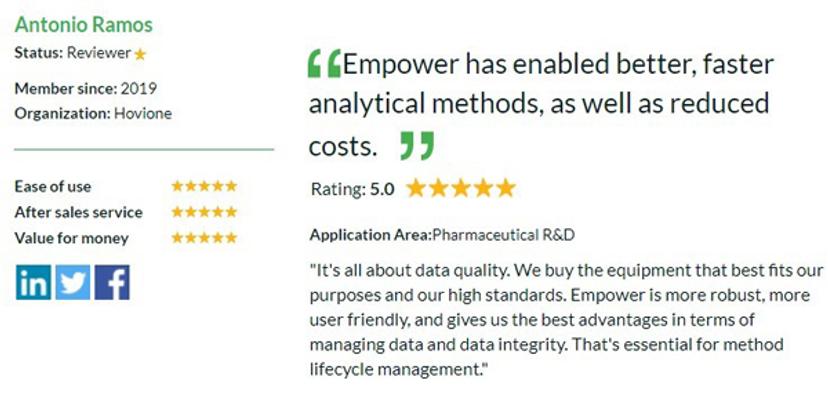
Take a look at what other researchers and scientists all over the world are saying about the latest equipment and technologies in drug discovery . Antonio Ramos, Hovione, shared his opinion on the Empower™ Chromatography Data System by Waters:
Find more resources on how to further your drug discovery research in our Advances in Drug Discovery Special Feature>>

