
Humanized mice are reshaping the biologic drug development pipeline

Ninety percent of drug candidates that enter clinical trials fail, largely due to a lack of clinical efficacy or unmanageable toxicity. Furthermore, developing a therapeutic typically takes over a decade and costs approximately $1 billion USD. To accelerate successful clinical outcomes and advance global human health, there is a critical need to expand the drug developers' toolbox with in vivo preclinical models that provide true translational data, especially for therapeutics with high human specificity.
Introducing humanized mouse models
These models, characterized by the introduction of human genes, cells, or tissues into murine hosts, represent the next generation of tools for preclinical immunotherapy drug development. Unlike conventional drug development models, humanized mice enable early and accurate identification of problematic compounds, fine-tuning of lead candidates to enhance efficacy and reduce toxicity, and reliable clinically predictive data to better inform clinical trial design and starting dose. Leading pharmaceutical entities are increasingly utilizing humanized mice in their drug development pipelines to increase the likelihood of efficacious clinical outcomes.
Advantages of humanized mouse models
- Improved clinical relevance: Humanized mice reliably recapitulate human physiology and pathophysiology, enabling better predictions of critical aspects of the human response to immunomodulatory drugs.
- Flexibility and customization: Humanized models are designed to offer flexible customization, allowing for more accurate modeling of various diseases and testing of different therapeutic types and mechanisms of actions (MOA).
- Practical benefits: Humanized mice are easily maintained in controlled laboratory settings, reducing reliance on non-human primates, ultimately saving time and resources.
The Jackson Laboratory (JAX), a biomedical research institute, is committed to supporting drug discovery by developing and providing access to humanized mouse models. JAX also offers in vivo preclinical drug evaluation services that use humanized models to better inform and accelerate drug development. Below, we delve into some of JAX’s humanized models and in vivo services.


FcRn mouse models: Reliable prediction of clinical pharmacokinetics
A daunting challenge facing clinical drug developers is selecting the starting dose, especially for compounds that have not yet been administered to humans. This selection is based on several preclinical parameters, including in vivo efficacy, toxicity, and pharmacokinetic (PK) data.
However, obtaining reliable and clinically predictive readouts for these parameters is challenging, particularly for therapeutics with high human specificity. While this specificity is advantageous in reducing off-target effects in humans, it renders the therapeutic inactive in conventional animal models making the data from these models inaccurate and unreliable.
To address this issue, JAX has developed a Humanized Pharmacokinetics (HuPK™) platform that enables the precise prediction of the human PK profile of therapeutic antibodies with accuracy comparable to that of non-human primate models. The HuPK™ platform uses JAX’s genetically humanized FcRn mouse models, which exclusively express human FcRn, an important recycling mechanism for extending serum half-life.
JAX has several FcRn model variants to optimize both PK and lead candidate selection assessment based on therapeutic type and MOA. Learn more about JAX’s FcRn model variants and HuPK™ services through the resources below.



Immune humanized and Onco-Hu® mouse models: Customizable efficacy testing
Efficacy testing is crucial in drug development, as it validates the therapeutic potential of candidates and guides fine-tuning before human trials. While conventional murine and in vitro models have been instrumental in advancing our understanding of biology and immune function, the significant differences between murine and human immune systems have posed a substantial challenge in extrapolating positive therapeutic outcomes from these models to the clinic.
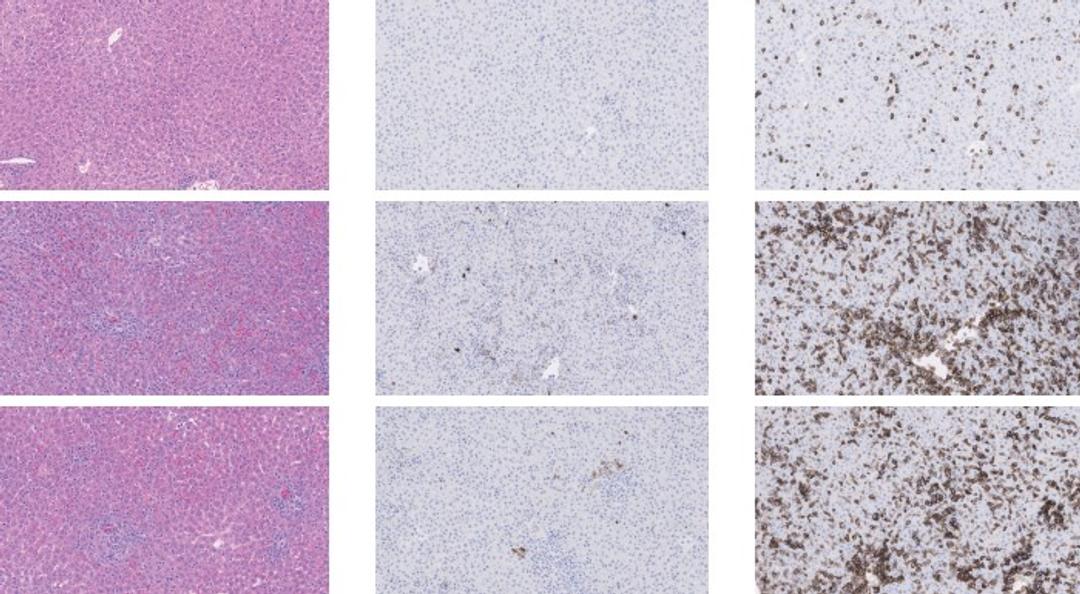
JAX’s immune humanized NSG, NRG, NSG-MHC I/II DKO, and Onco-Hu® models (mice dually engrafted with a human immune system and xenograft) help overcome this translational gap. By supporting engraftment and maturation of human immune cells and tumors, these models reliably recapitulate human physiology, pathophysiology, and tumor microenvironment to predict how a therapeutic will behave in the clinic.
In addition, unlike conventional models, the modular nature of humanized mice permits flexibility in both the humanization approach (CD34+ hematopoietic stem cells or peripheral blood mononuclear cells (PBMCs)) and in xenograft source (cell line-derived or patient-derived). This flexibility establishes an efficacy testing platform that can be tailored to test each therapeutic type in its most applicable environment, enabling efficient and thorough evaluation.
JAX’s immune humanized and Onco-Hu® mice are available for procurement or immediate enrollment in JAX’s Drug Efficacy Testing Services. Below are resources to help with humanized mouse model selection as well as data from Drug Efficacy Case Studies using humanized mice.


Humanized NSG mice are used by researchers and drug discovery scientists as powerful tools to study hematopoiesis, inflammatory disease, host-pathogen interactions, and the development of novel therapies for infectious disease. Understanding the development of humanized mice and how to select the appropriate model are important considerations for conducting impactful research using humanized mice.
Download resource
PBMC humanized mice: Ensure the safe progression of promising therapies into clinical trials

Safety testing is a vital process in the drug development pipeline, serving to mitigate toxic effects that nullify the benefits of treatment, safeguard patient well-being, secure regulatory approval, and uphold public trust. Since most immunotherapeutic drugs activate the immune system, they risk triggering an overactive immune response, usually by inducing an excessive cytokine release. Iatrogenic cytokine-release syndrome (CRS) is recognized as one of the most common and dangerous adverse effects of almost all immunotherapies currently in development.
Drug developers face two significant hurdles when predicting CRS: the lack of therapeutic cross-reactivity with non-human species and large patient variability. JAX’s Acute Immunotoxicity Evaluation Platform addresses both issues to assess the safety profile of a therapeutic more accurately and reliably. The platform uses pre-characterized PBMC humanized mice that can be co-engrafted with a tumor. These models overcome the specificity issue by expressing human immune targets. And, by utilizing cohorts of mice engrafted with different PBMC donors, the platform can mirror the large patient variability observed in the clinic, thereby providing a more comprehensive model for predicting CRS.
Explore JAX’s resources below to learn more about CRS and review CRS case studies.


Stay informed on JAX products, services, and educational content
The monthly e-newsletter, JAX Pulse, is the best way to stay up-to-date on JAX's services, models, webinars, and educational content.
Sign up for free to:
- Learn about the newest, cutting-edge in vivo services and mouse models
- Meet the scientists at JAX and find out what drives them to search for tomorrow’s cures
- Be the first to know about upcoming webinars, educational courses, workshops, and events
