Agilent Cary WinUV Dissolution Software
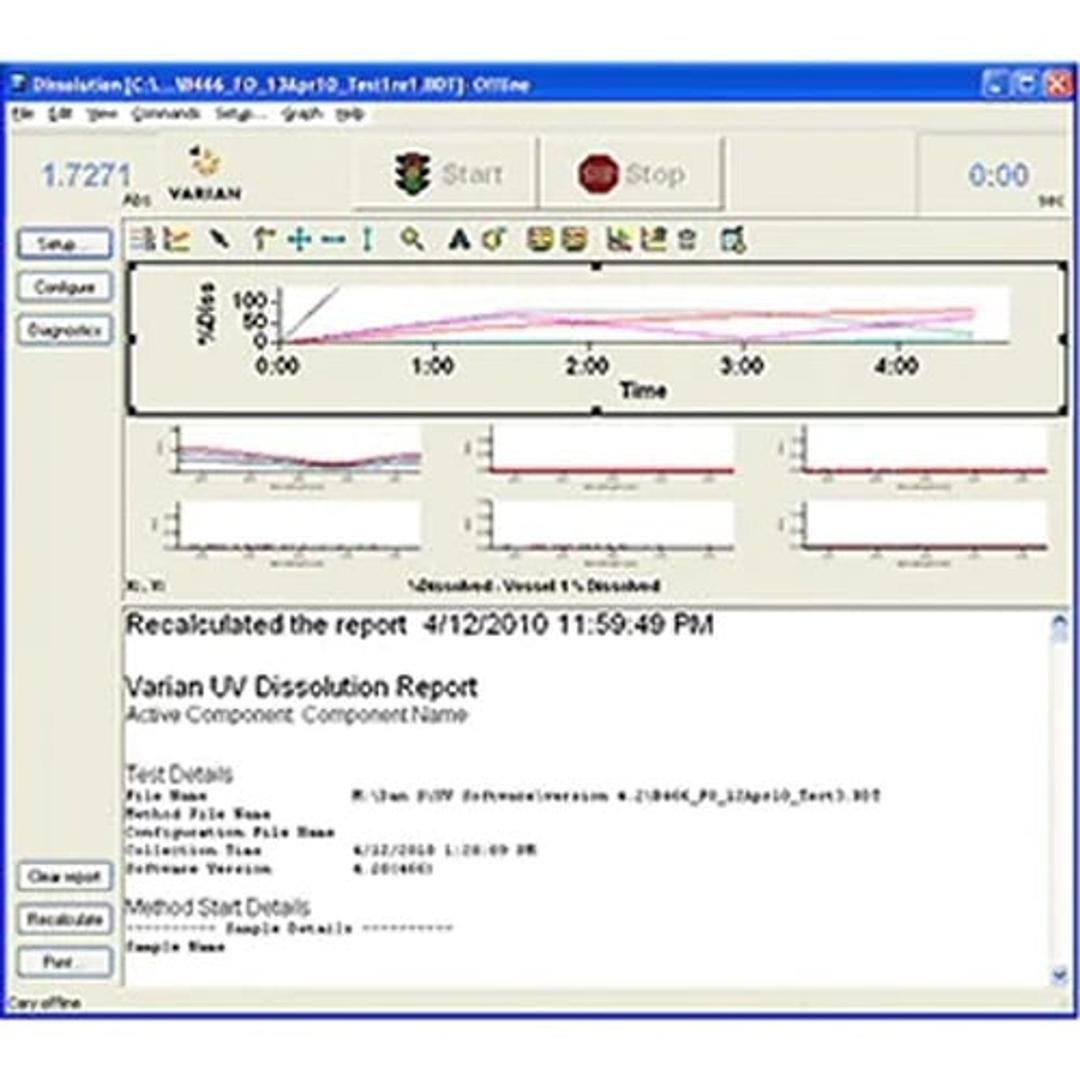
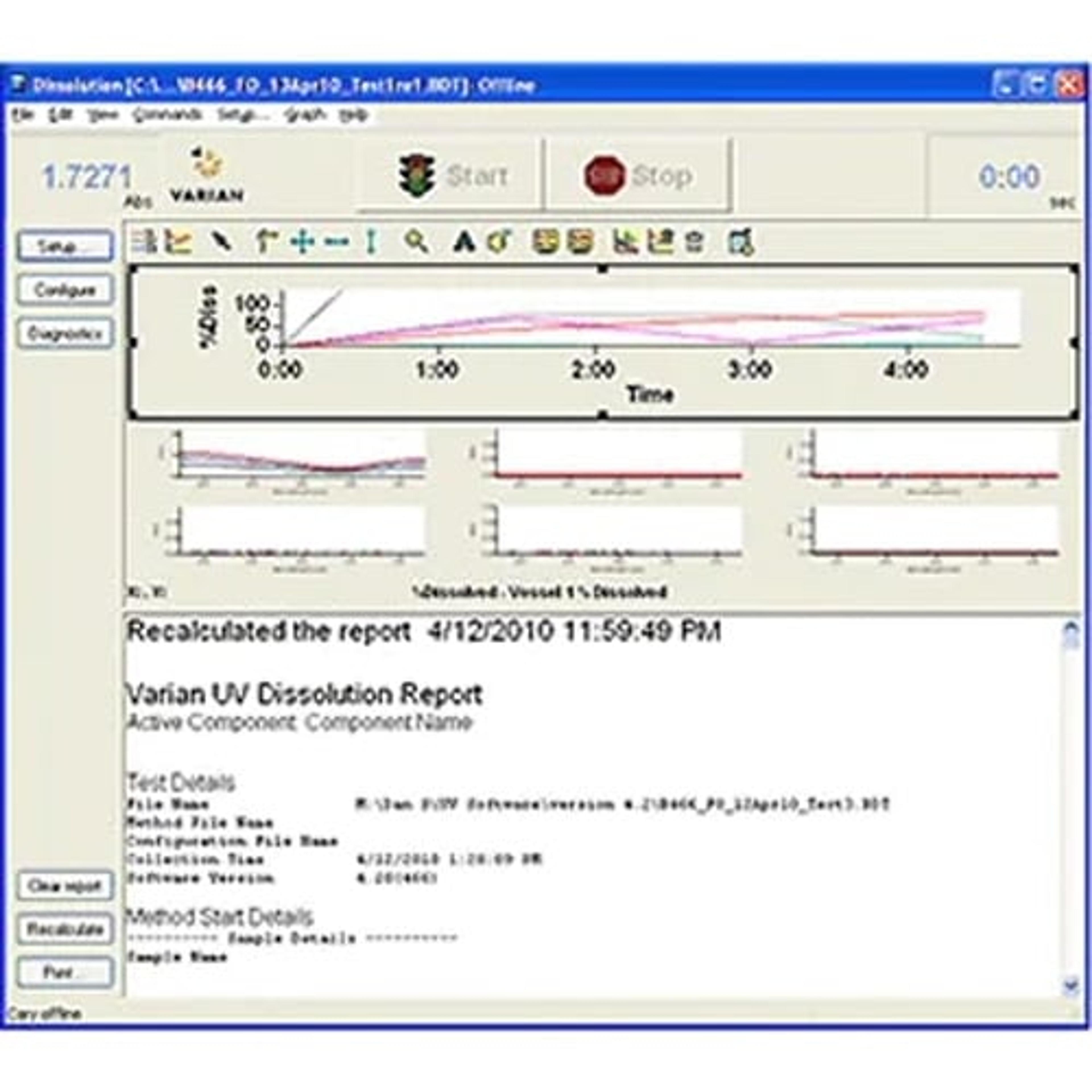
Cary WinUV dissolution software supports both Agilent multicell and fiber optic online UV dissolution systems. The software generates accurate and reliable data and accommodates a wide variety of dissolution samples and methods. Analysts can easily customize final reports with a complete summary of the data acquisition using comparison and statistical evaluation tools, data tables and dissolution profiles.
Super instrument, could not imagine my Ph.D. without it. My best friend in my Ph. D.
analyze drug properties
Product is very effective and very easy to use. It's easy to maintain and gives sharp results. Has good software, fast scan process and gives great reproduciility.
Review Date: 16 Mar 2021 | Agilent Technologies
Cary WinUV dissolution software supports both Agilent multicell and fiber optic online UV dissolution systems. The software generates accurate and reliable data and accommodates a wide variety of dissolution samples and methods. Analysts can easily customize final reports with a complete summary of the data acquisition using comparison and statistical evaluation tools, data tables and dissolution profiles.
The software is designed to work in a 21 CFR Part 11 environment.
Features:
- Integrate Cary WinUV dissolution software with a peristaltic or syringe pump, filter changer, and 8000 sampling station for accurate preparation of samples and archival
- Control apparatus features such as dosage delivery, automated sampling, and vessel temperature monitoring
- Create data processing and reports for samples taken offline using the UV dissolution manual program
- Perform media change methods, capsule shell corrections, and infinity spins required for various methods
- Work with challenging spectra, including advanced UV capabilities, such as second derivative analysis, baseline correction, and more
- Built-in technical controls ensure the security of your data, control access, and facilitate compliance as defined by US FDA 21 CFR Part 11, EU Annex 11 and similar national electronic record regulations