Agilent Dissolution Workstation Software
Agilent dissolution workstation software integrates dissolution apparatus and automated sampling components to simultaneously control up to four systems of any configuration from a desktop PC.
Agilent dissolution workstation software integrates dissolution apparatus and automated sampling components to simultaneously control up to four systems of any configuration from a desktop PC.
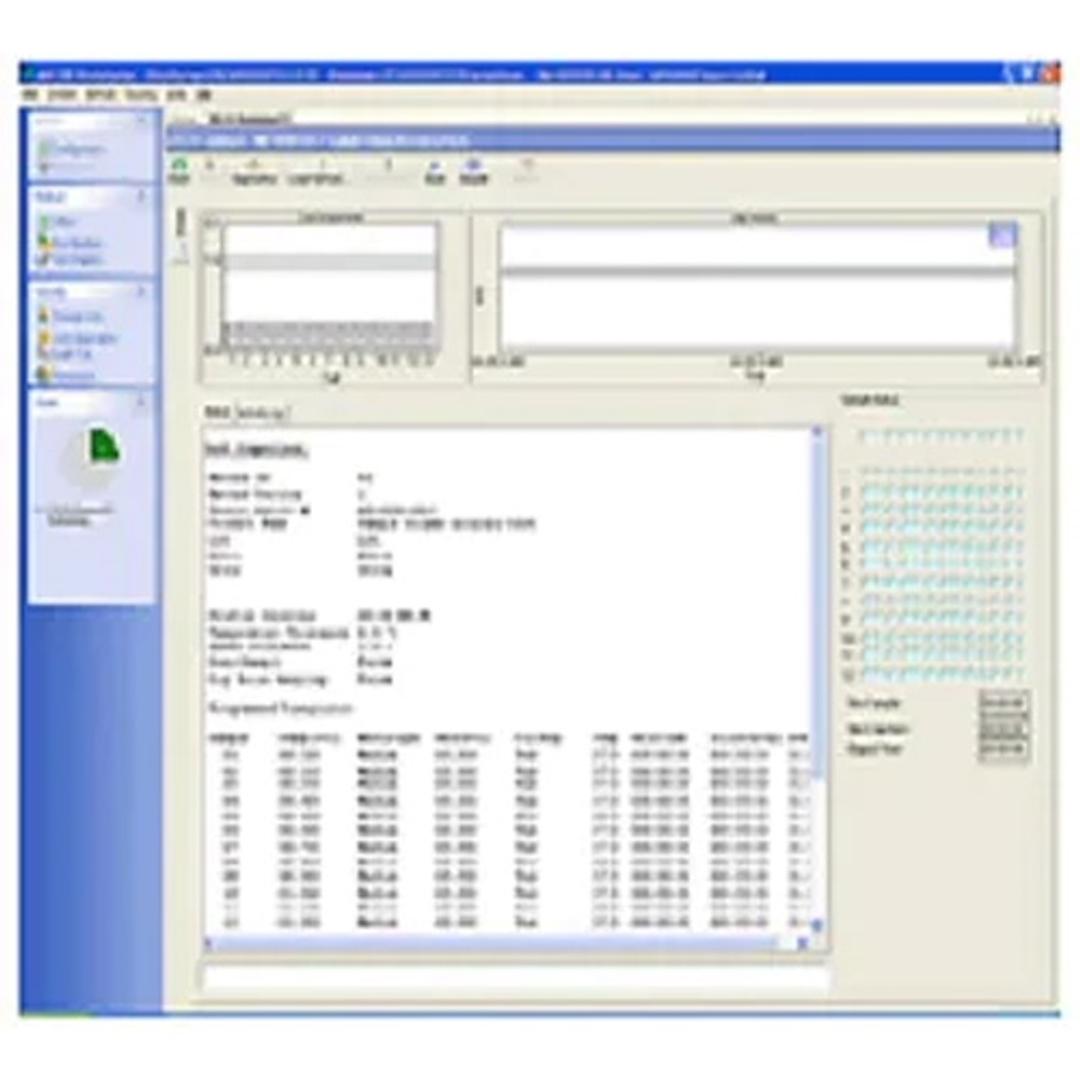
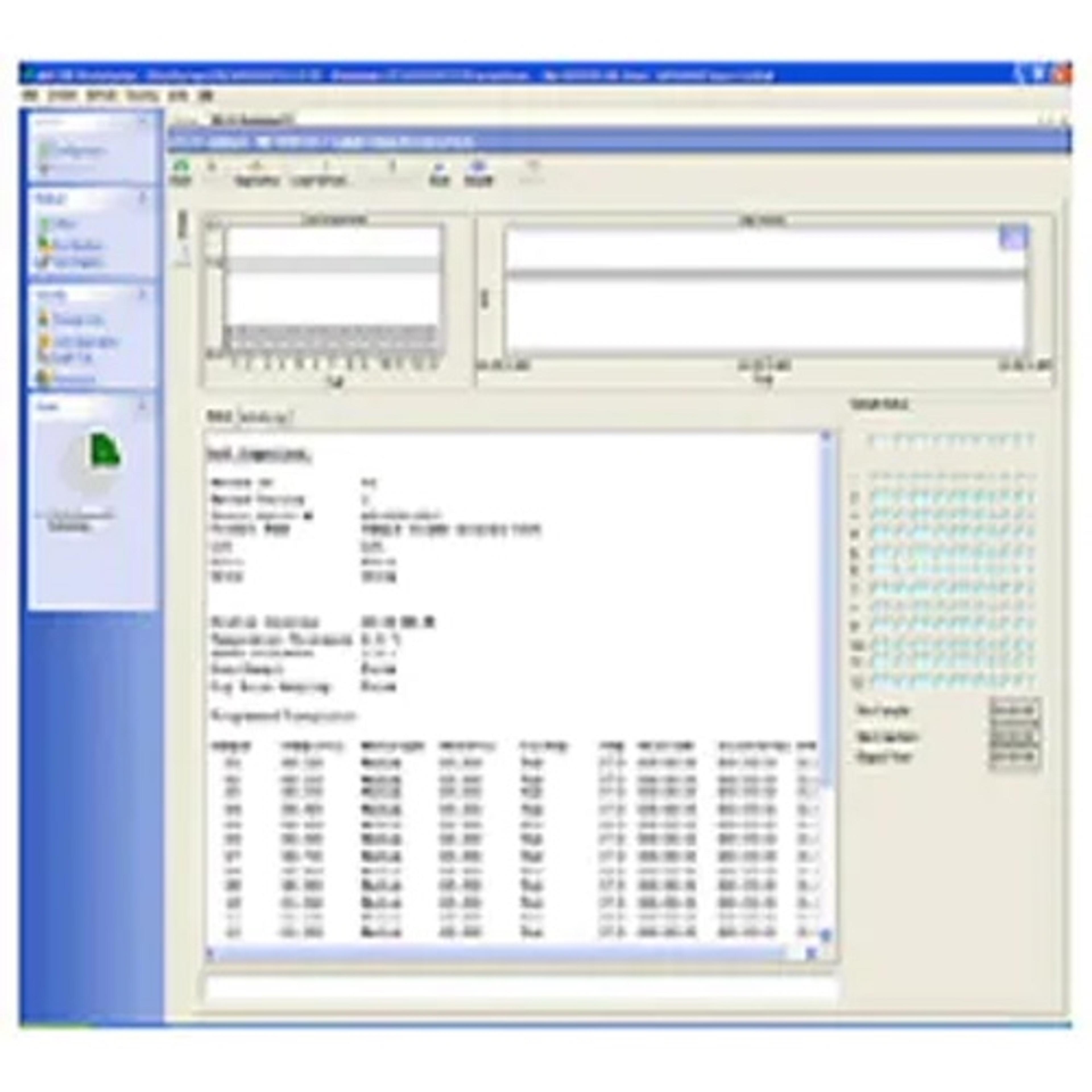
The software lets you build, edit, search, retrieve, and archive all dissolution methods and test reports from a single interface. Method parameters, instrument/accessory information, and test data is captured and recorded. Test status is visible in real time as it progresses through the timepoints for each dissolution system. User changes can also be tracked as part of 21 CFR Part 11 compliance.
Features:
- Monitor methods in real time, as well as capture and record method parameters, temperature measurements, and sampling times
- Control of up to four (4) dissolution systems independently from a single PC
- Track all method/system updates as well as tests performed and store them in easily searchable archives
- Print, preview, or export documented results generated by a dissolution run in the protected database created locally or on a network
- Maintain a complete history for all executed tests, which can be digitally verified and electronically signed
- Integrate or export the data to a laboratory information management system (LIMS) through the client-server architecture
- Control user rights and privileges by leveraging the security capabilities of the Windows operating system
- Use with all Agilent dissolution instrumentation
- Reduce paper trail and manual transcription errors associated with standalone or semi-automated systems
- Built-in technical controls ensure the security of your data, control access, and facilitate compliance as defined by US FDA 21 CFR Part 11, EU Annex 11 and similar national electronic record regulations