Products & ReviewClinical Diagnostics
EURORealTime APOE and Alzheimer’s Biomarker Panels
EuroimmunAvailable: Worldwide
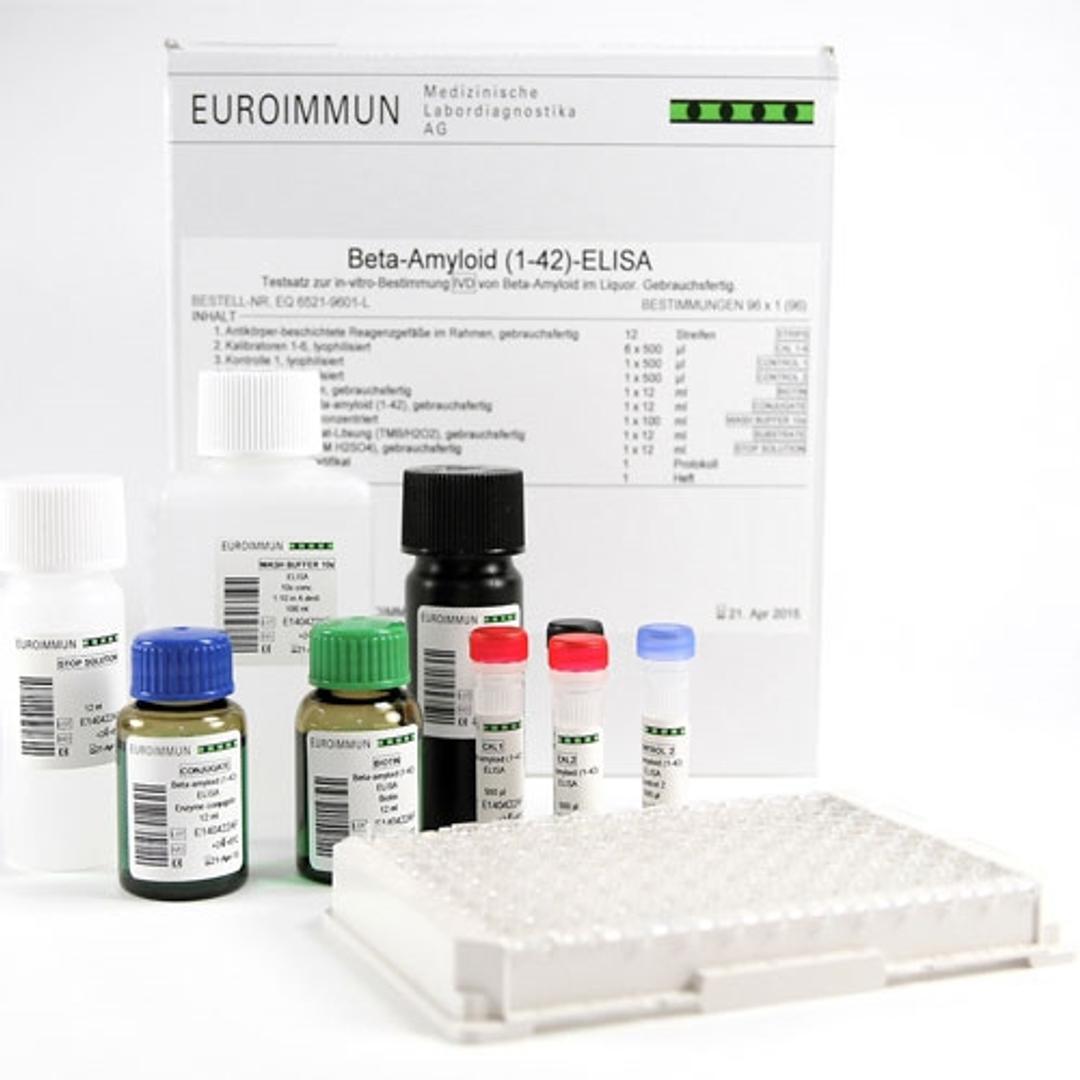
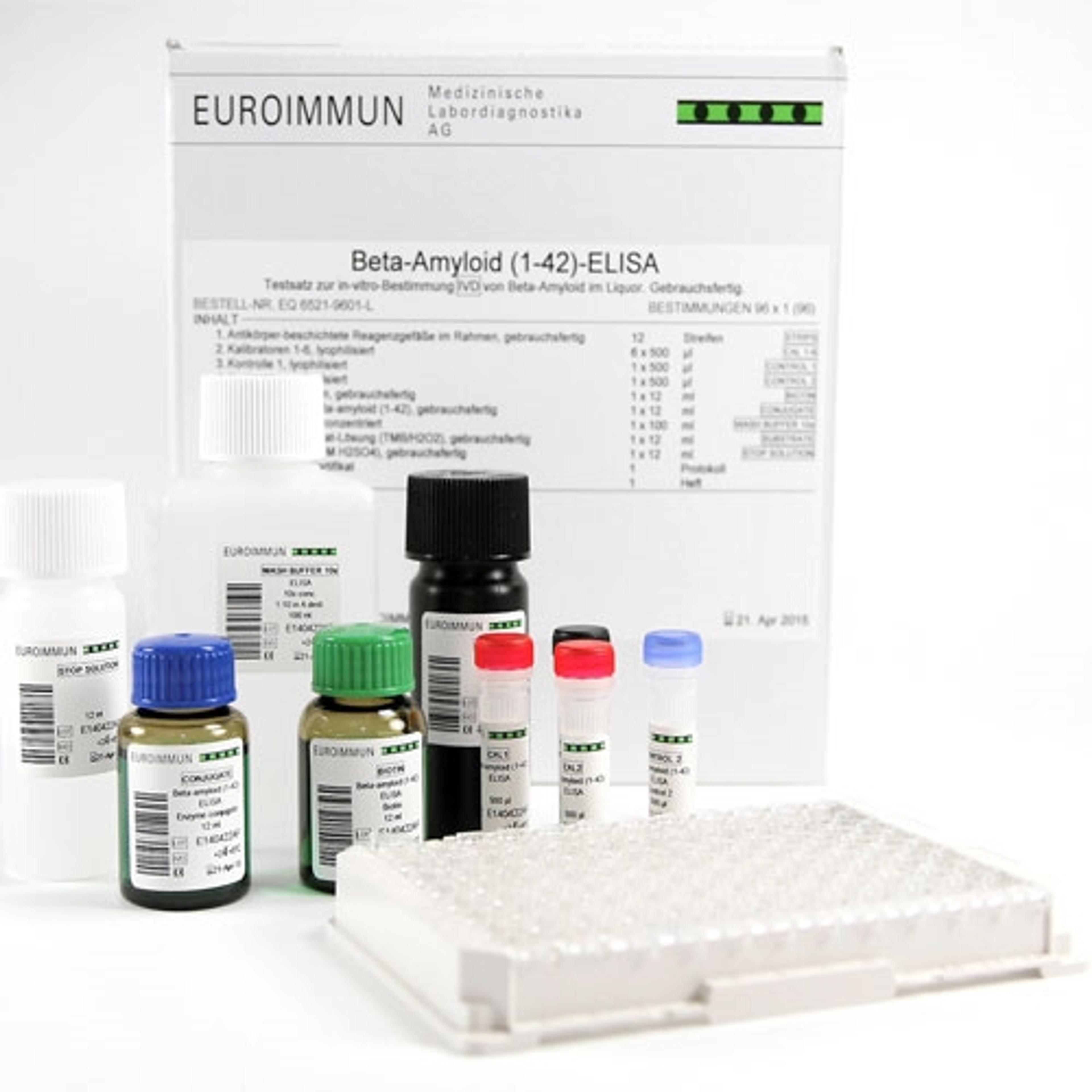
The EURORealTime APOE enables precise determination of the APOE genotype to support risk assessment prior to anti-amyloid therapy for Alzheimer’s disease. The test includes automated data analysis for easy result readout. Only one reaction is required per sample. To support early diagnosis of the disease, Euroimmun offers biomarker assays for robust measurement of beta amyloid, total tau and p-Tau(181) in CSF.
EURORealTime APOE
- Precise genotyping of the six APOE genotypes: ε2/ε2, ε2/ε3, ε2/ε4, ε3/ε3, ε3/ε4 and ε4/ε4
- Supports risk assessment prior to implementation of anti-amyloid therapy
- Guides therapy decisions, especially for ε4/ε4 homozygotes, who are excluded from anti-amyloid therapy in some countries
- One-tube reaction with genomic DNA isolated from EDTA blood
- Simple result readout due to automated data analysis by the software EURORealTime Analysis
Biomarker test panels
- Precise measurement of beta amyloid 1-42/1-40 ratio, total tau and P-tau(181) in CSF
- High specificity due to use of specific monoclonal antibodies
- Parallel processing of parameters using uniform protocols
- ELISA and chemiluminescence immunoassay (ChLIA) formats available with CE mark
- Fully automatable on established instruments
*Regulatory status, precise intended use and product availability must be verified for the user‘s individual jurisdiction