BiopharmaLynx
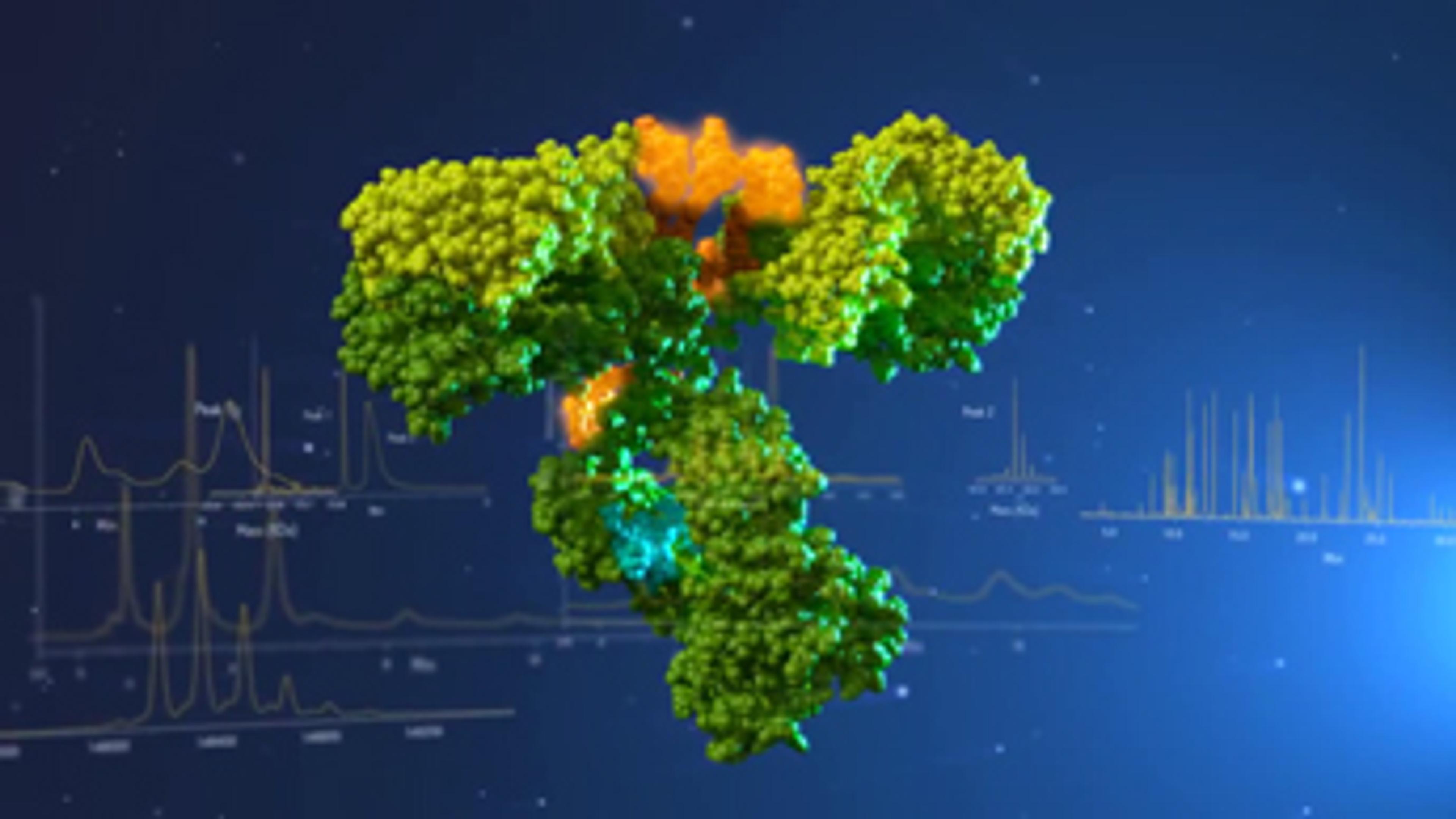
BiopharmaLynx™ automates the processing and interpretation of biopharmaceutical product LC/MS data, enabling you to characterize your products. Developed with biopharmaceutical thought leaders for confident characterization and optimized workflow, BiopharmaLynx™ leverages Waters' leading protein informatics expertise. Determine the identity, purity and stability of biopharmaceuticals, working with either peptide maps or intac…
BiopharmaLynx™ automates the processing and interpretation of biopharmaceutical product LC/MS data, enabling you to characterize your products.
Developed with biopharmaceutical thought leaders for confident characterization and optimized workflow, BiopharmaLynx™ leverages Waters' leading protein informatics expertise. Determine the identity, purity and stability of biopharmaceuticals, working with either peptide maps or intact proteins.
BiopharmaLynx Features:
- Automatically process your accurate mass MS data, including peptide map results and intact mass measurements.
- Automatically analyze and assign results, define the sequence and features of known proteins and determine the identity of modified forms.
- Allow your users to edit assignments, annotate new peaks and compare experimental samples with a reference using visualization and tabular tools.
- Automatically generate biopharmaceutical-specific reports from templates.
- Identify and quantify ions in a peptide map, including intra- and inter-sample relative quantitation.
- Assign ions to expected and modified peptides from a defined protein sequence, determining sequence coverage and ID-modified peptides.
- Define deconvolution settings for known proteins and as generic for unknown proteins.
- Annotate deconvoluted mass data with protein identities and their modifications.
- Automatically calculate percentages of each protein variant.