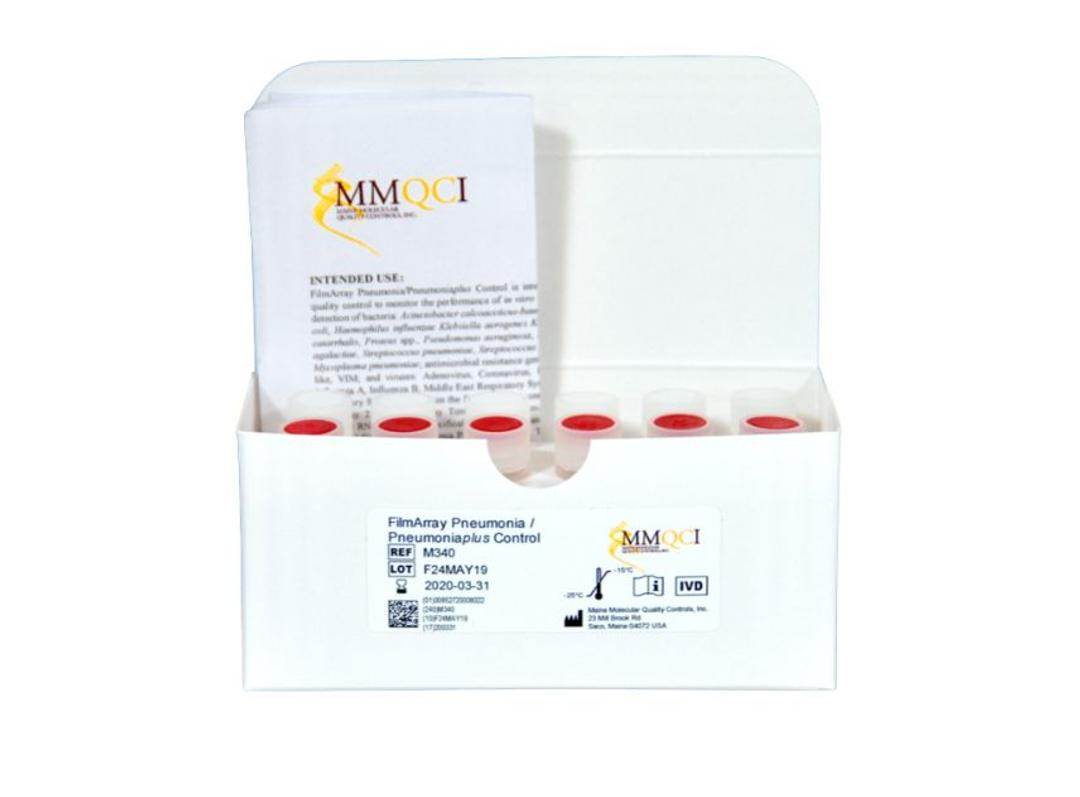
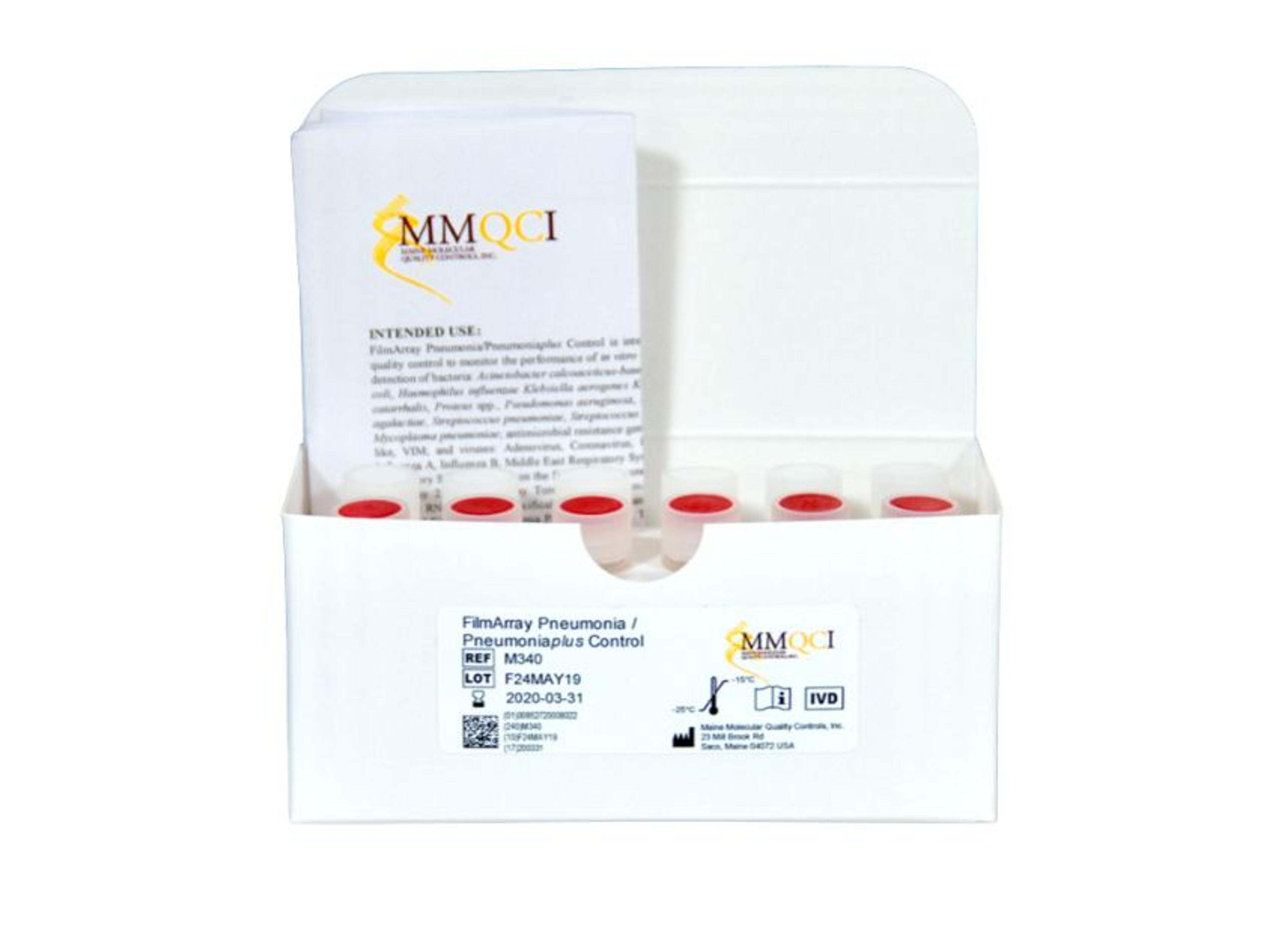
FilmArray® Pneumonia/Pneumoniaplus Control
FilmArray® Pneumonia/Pneumoniaplus Control is intended for use as an external positive and negative assayed quality control to monitor the performance of in vitro laboratory nucleic acid testing procedures for the qualitative detection of bacteria, antimicrobial resistance genes, and viruses included in the FilmArray® Pneumonia Panel or Pneumonia Panel plus assay performed on the FilmArray® systems.

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
FilmArray Pneumonia/Pneumoniaplus Control is composed of 2 controls, FilmArray® Pneumonia/Pneumoniaplus POSITIVE and FilmArray® Pneumonia/Pneumoniaplus NEGATIVE. FilmArray Pneumonia/Pneumoniaplus POSITIVE contains surrogate control material composed of synthetic nucleic acid corresponding to genome segments of pathogens listed in Table 1. FilmArray Pneumonia/Pneumoniaplus NEGATIVE contains no RNA or DNA. FilmArray Pneumonia/Pneumoniaplus Control is composed of synthetic RNA and DNA specifically designed for and intended to be used solely with the FilmArray Pneumonia Panel and FilmArray Pneumonia Panel plus assays. This product is not intended to replace manufacturer controls provided with the device.