Luminata
Enterprise decision support software for pharmaceutical and chemical development. The only software that consolidates your process and analytical data in one place.
The program is very useful.
Pharmaceuticals, stress testing
We use luminata software. the program is very useful.
Review Date: 12 Mar 2020 | Advanced Chemistry Development, Inc., (ACD/Labs)
Easy to use, great support.
Pharmaceuticals, stress testing
Easy to use, great support.
Review Date: 12 Mar 2020 | Advanced Chemistry Development, Inc., (ACD/Labs)
Let Scientists Focus On Science
The future of science is collaborative. Cutting-edge pharmaceutical and chemical development involves sharing information within a multidisciplinary team who track many types of analytical and process data.
Your data should be useful. More sources of data means more spreadsheets. More incompatible files. More time spent tracking down results. Before long, data management gets in the way of research and decision-making.
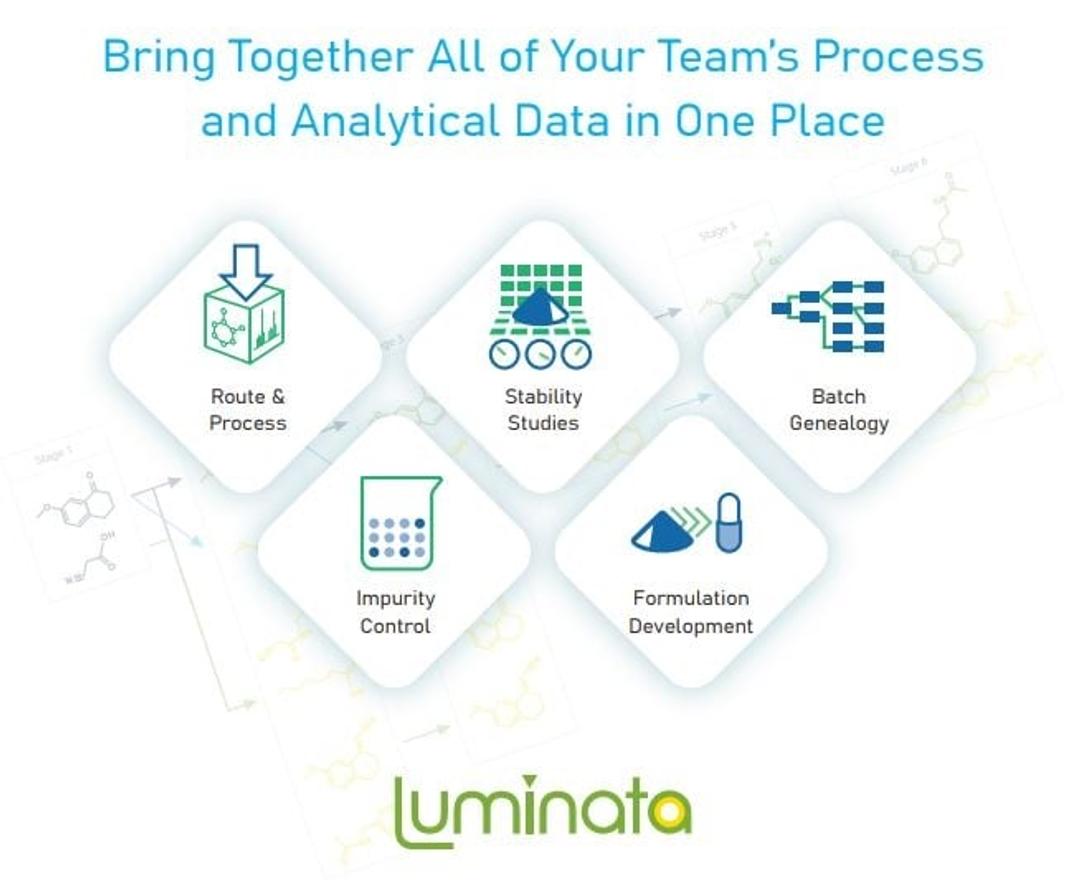
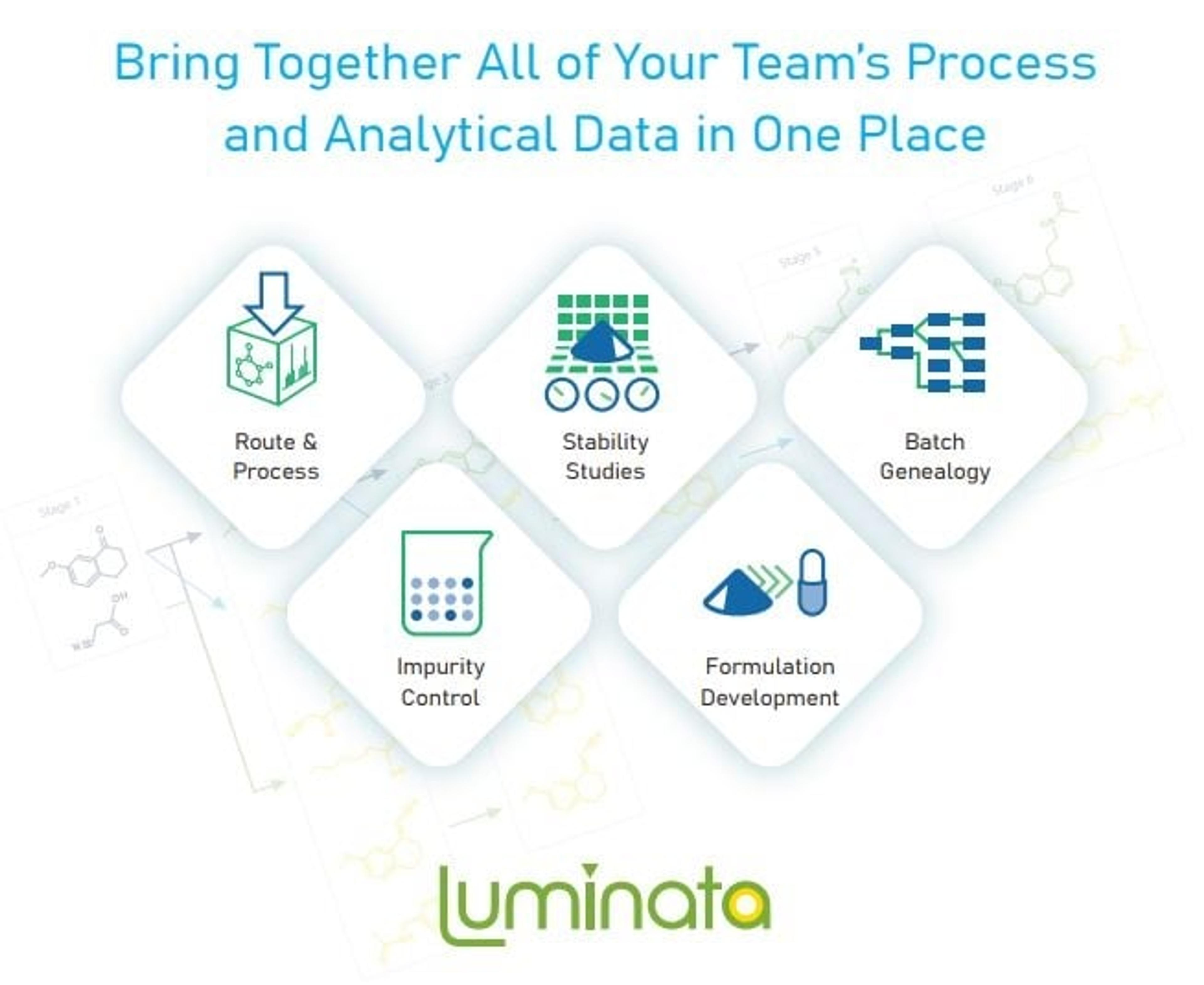
Make your data work for you with Luminata. Luminata® is the only Chemistry, Manufacturing, and Controls (CMC) decision support software. It lets your team store, search, map, process, and reuse all your chemical and process data in one application.
The Data Management Solution for Your Entire Team
Analytical Chemistry
• Consolidate analytical data in one application that supports >150 data formats
• Connect analytical and process data
• Share analytical results with colleagues in real-time
Process Development and Control
• Improve reaction planning with process maps
• Simplify impurity management
• Build effective process control strategies
Team Leads and Management
• Track project progress across locations
• Assess batch data throughout the supply chain
• Accelerate regulatory submissions with report building tools
Route Scouting & Process Development
Consolidate all your process development information in one location. Connect analytical data with route schema to ensure a complete understanding of multi-stage process routes.
• Build comprehensive maps for your evolving process
• Link analytical data to each chemical in your scheme
• Integrate Quality by Design (QbD) principles in route selection
• Design a process map by importing LC/UV/MS datasets to drive creation of new entries, or select existing compounds from the onboard reference dictionary
• Categorize entries in the process scheme as Starting Materials, Intermediates, API(s), Impurities, Degradants, Solvents, or Reagents
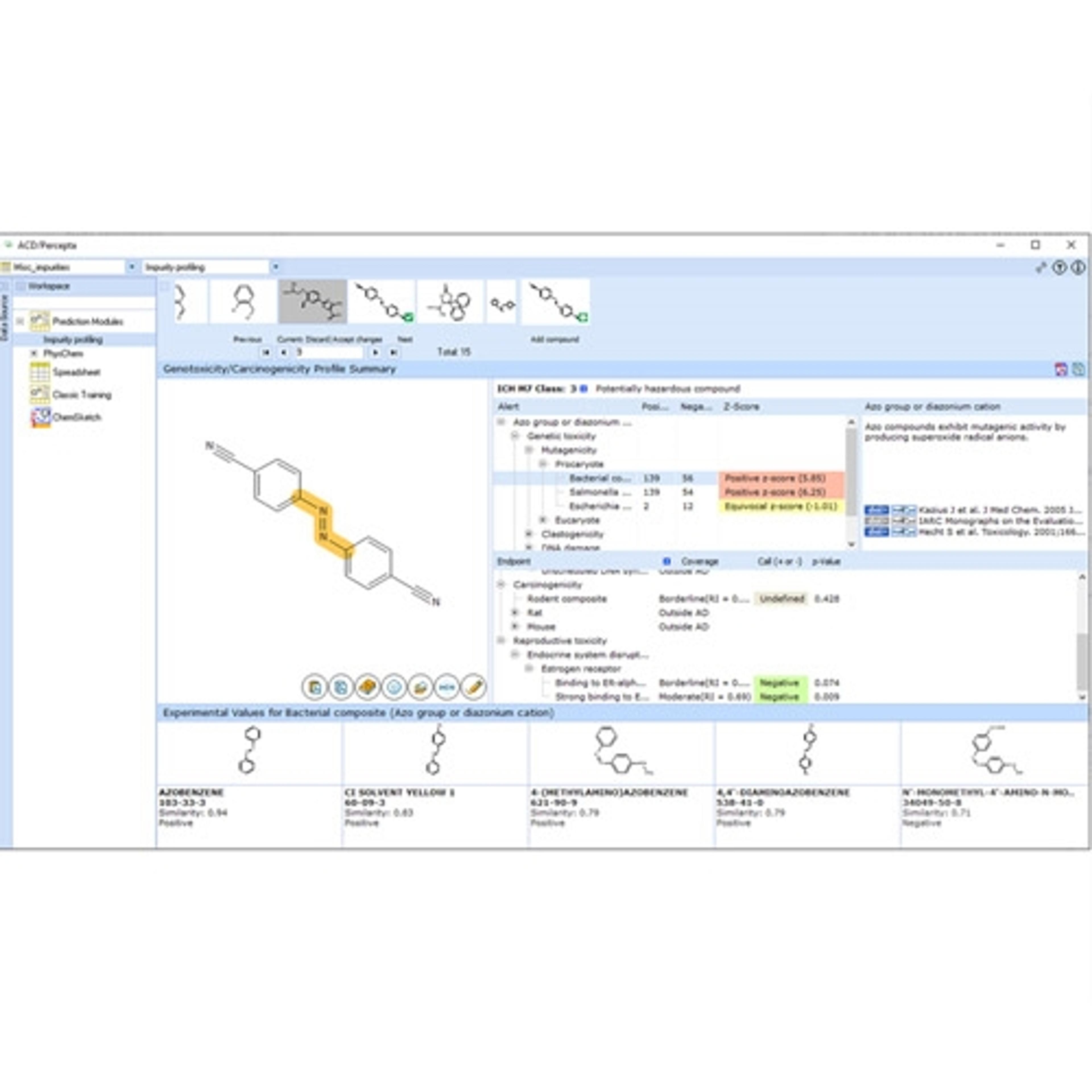
Impurity Control
Establish effective process and analytical impurity control strategies based on live spectral data and route knowledge, in an environment built for Quality by Design (QbD) in pharmaceutical drug development.
• Use an impurity map to automatically track fate and purge data
• Auto-calculate impurity carryover from analytical results
• Develop robust controls based on all the relevant data in one place
• Build control strategies through the impurity master mode functionality
• Perform fate & purge, and spiked impurity calculations
Drug Substance & Drug Product Stability
Assemble degradation maps that cover complete drug substance and drug product forced degradation studies.
• Import and process forced degradation HPLC and MS datasets for a complete study map
• Make formulation decisions based on kinetic plots automatically created from analytical data
• Search for target impurities across forced degradation experiments
• Automatically process time-point datasets to create a forced degradant map and kinetic plot(s) that capture all forced degradant information in one location
Formulation Development
Luminata’s drug formulation tools accelerate the transition from drug substance to drug product by integrating analytical and process data in one place.
• Develop design protocols based on the chemistry of the API / Drug Product formulation
• Build comprehensive formulation maps
• Perform drug-excipient comparability studies and test formulations
• Perform excipient case studies for preformulation studies
Supply Chain Analytical Data Management
Use Luminata’s supply chain data management tools to attach analytical data to each batch for real-time decision-making and batch genealogy tracking.
• Create a comprehensive family tree of every batch for a complete supply chain map
• Identify irregularities by comparing analytical data to a reference
• Use structure search to quickly determine the presence of an impurity/entity in all batches
• Do batch-to-batch comparison with analytical data
• Register and integrate all batches created internally and externally
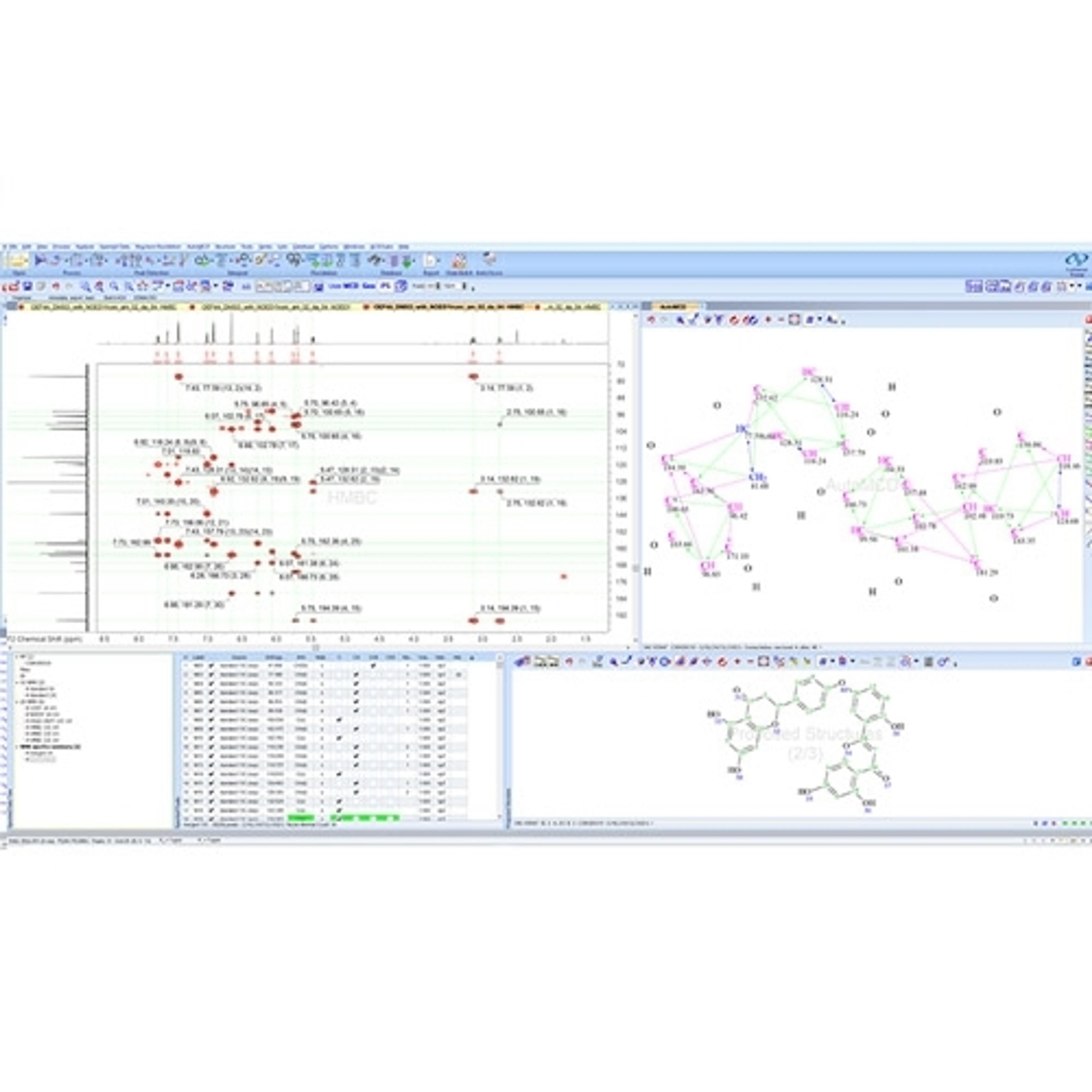
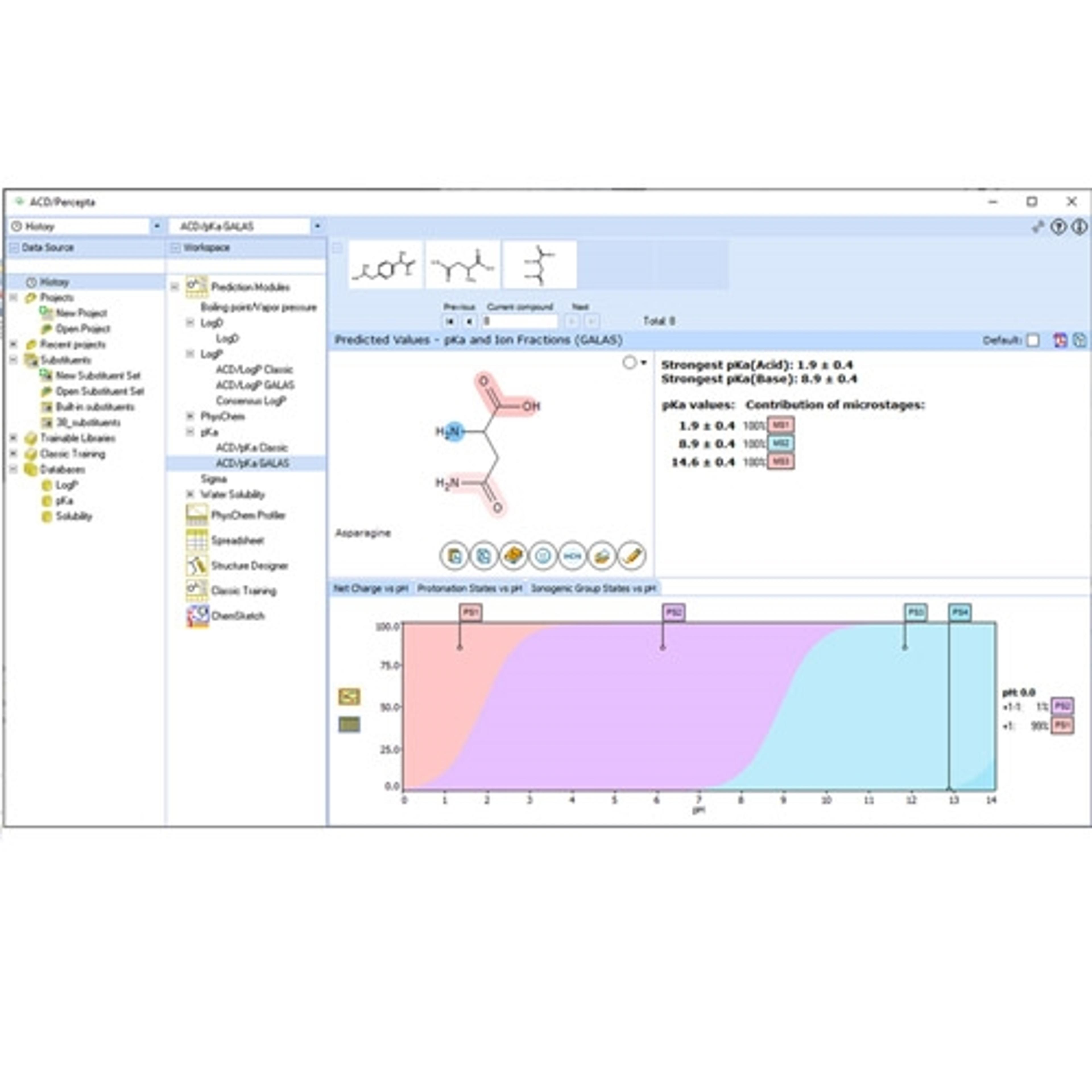
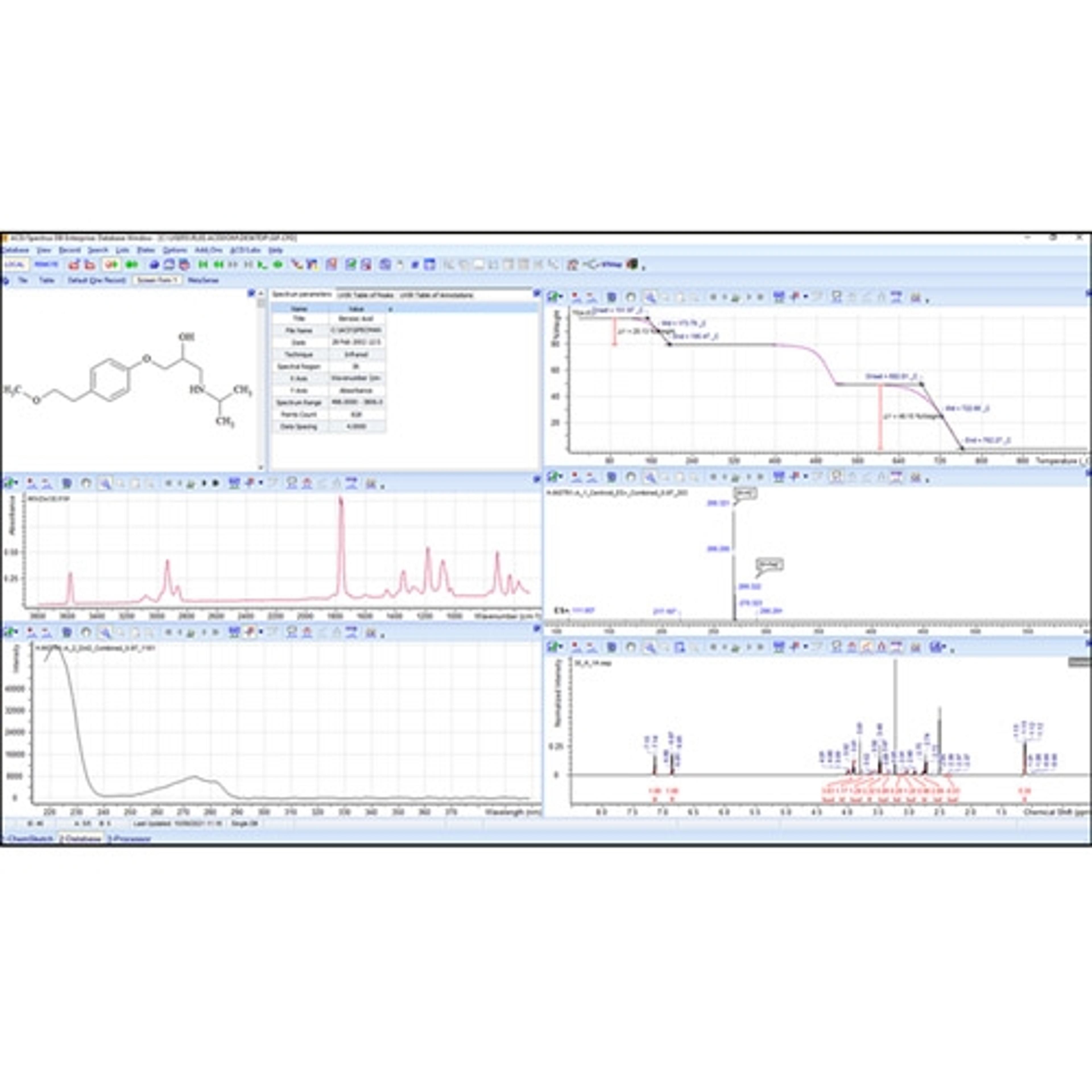
Data Import and Analysis
Luminata is built on the Spectrus platform, allowing you to import, process, and store your analytical data.
• A single data format for the analytical techniques used to characterize compounds and formulations in your labs. LC/MS, GC/MS, NMR, Chromatography, Raman, IR, UV, Visible, and more
• Native support for >150 data formats including major instrument vendors, open-source formats, and emerging standards (e.g., AnIML, JCAMP, and ADF from Allotrope).
• Luminata will detect the type of data you’re importing and offer tools to process and analyze it.
• Connect and assign full chemical and biochemical structures, structure fragments, Markush structures, or atoms to spectra and chromatograms.