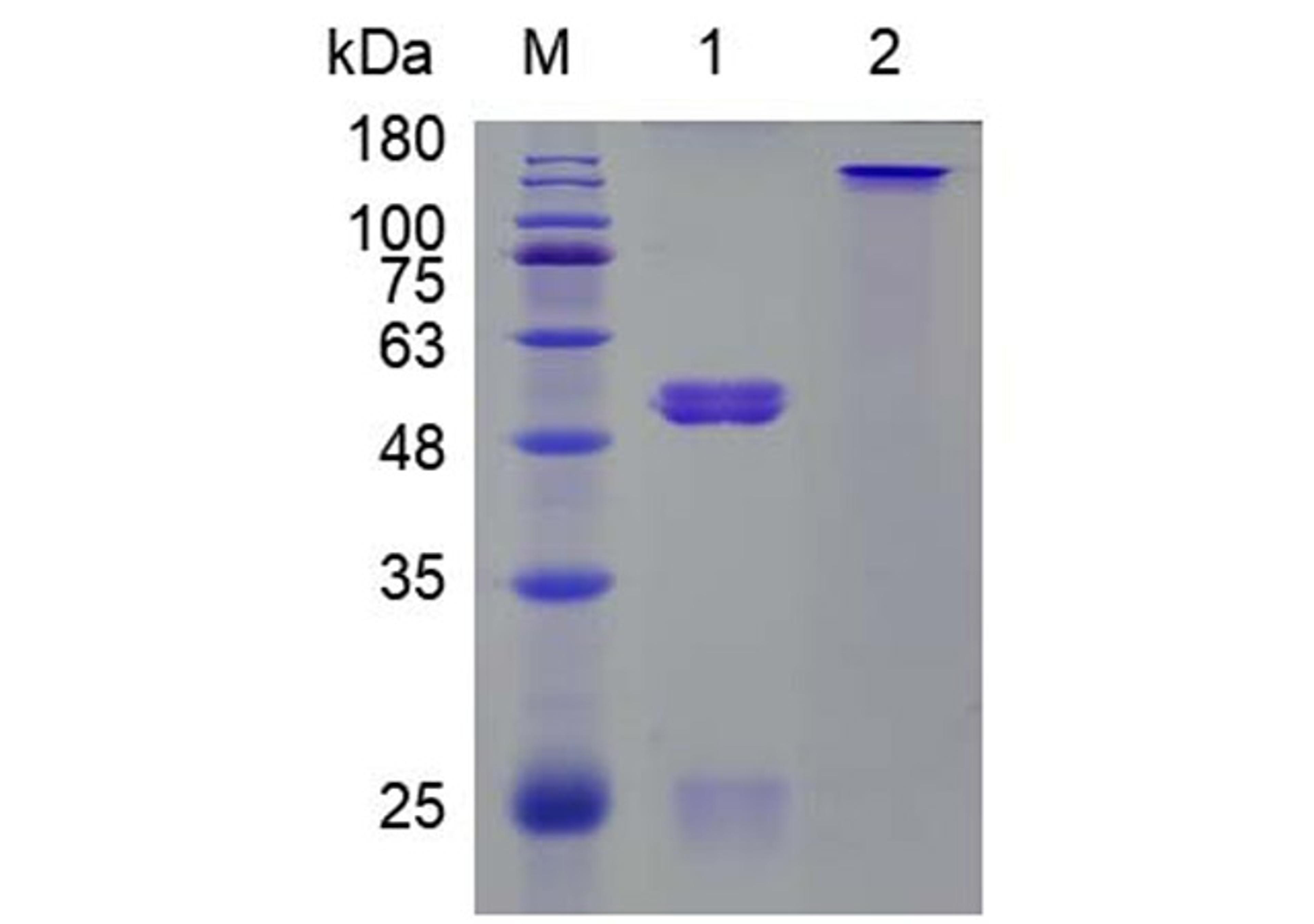
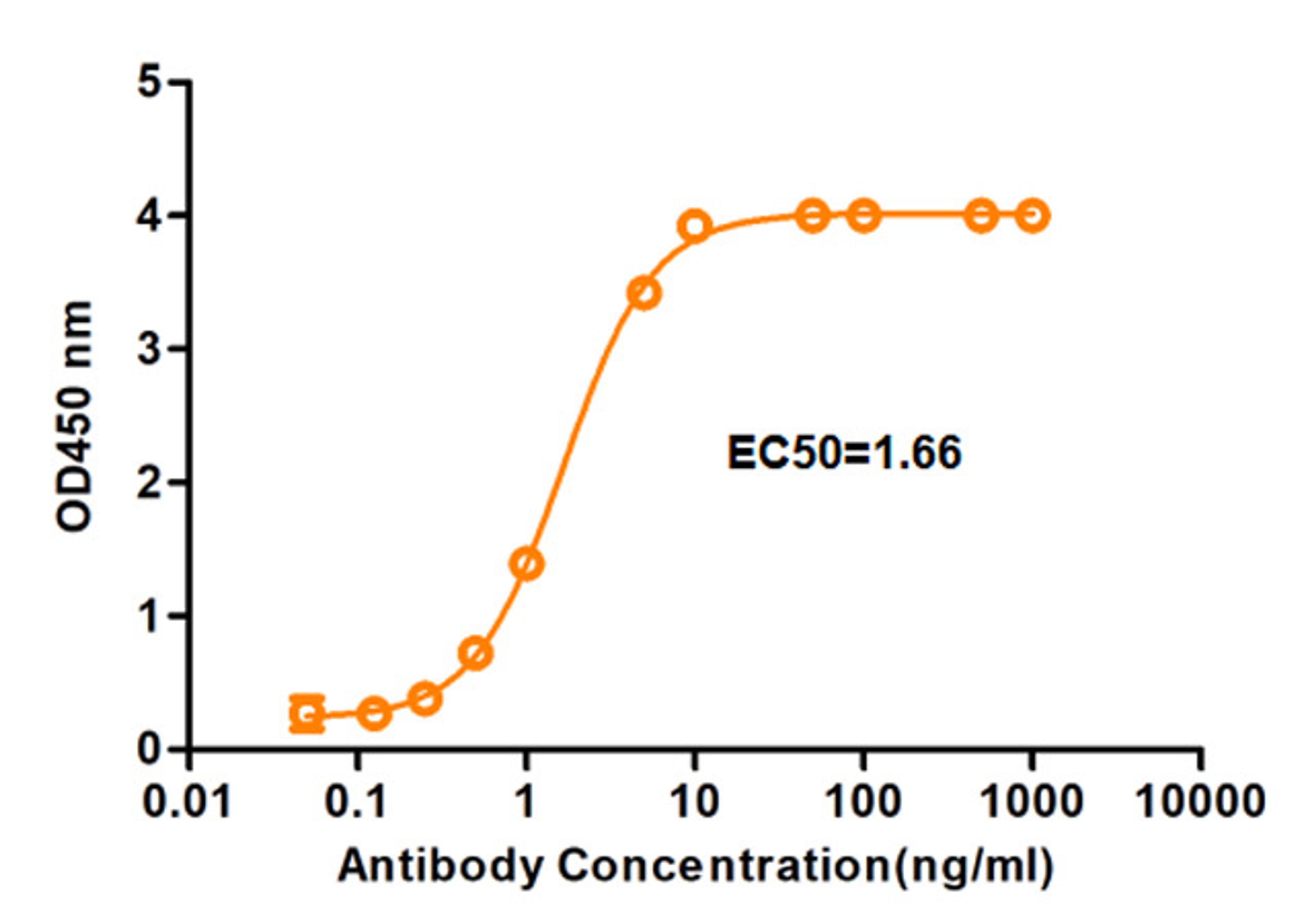
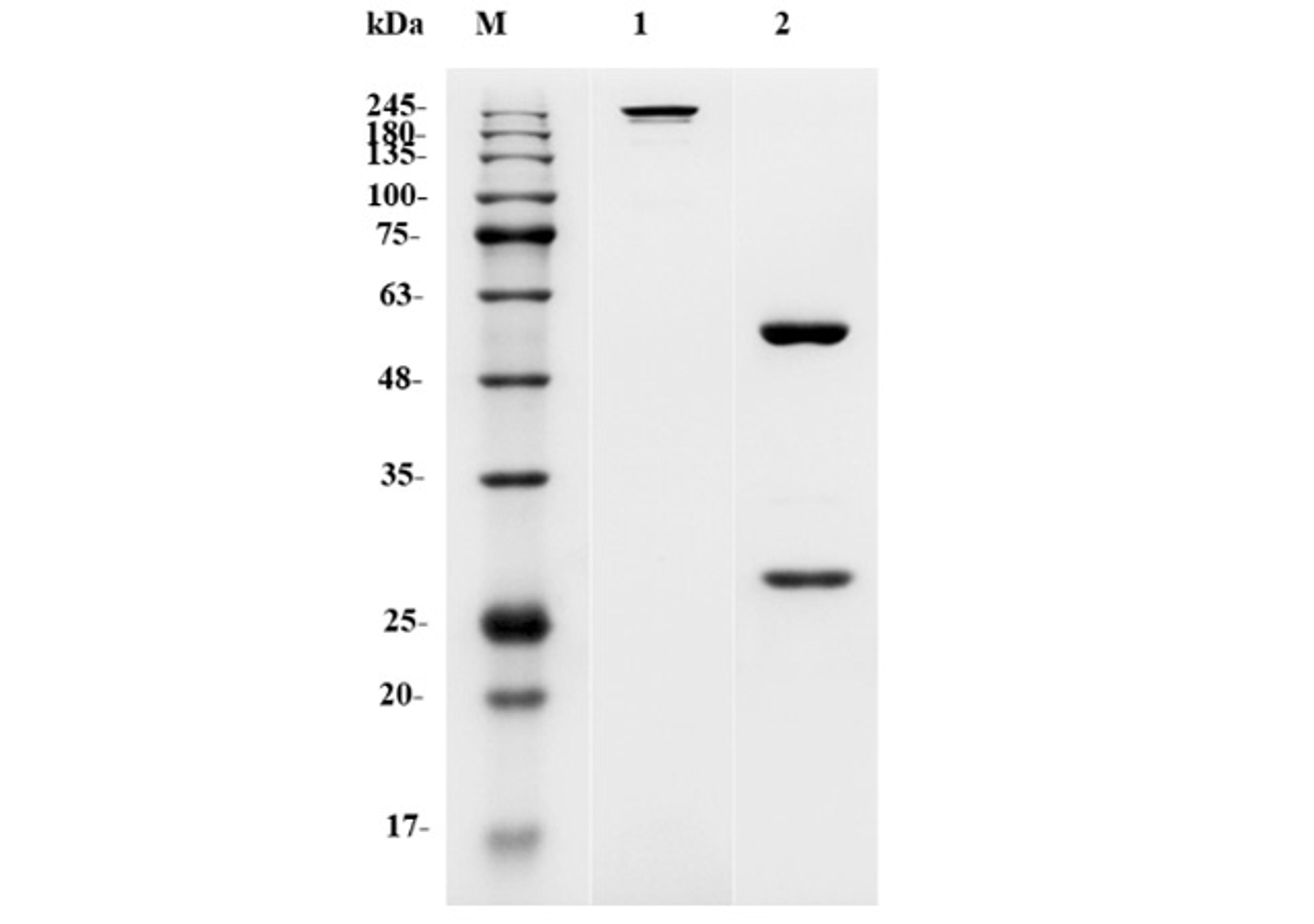
MetCombi
High Quality Assays with Reproducible and Reliable Results

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
Enzyme Immunoassay for the quantitative determination of Metanephrine and Normetanephrine in urine.During the sample preparation Metanephrine (Metadrenaline) and Normetanephrine (Normetadrenaline) are quantitatively acylated. The subsequent competitive ELISA kit uses the microtiter plate format. The antigen is bound to the solid phase of the microtiter plate. The acylated standards, controls and samples and the solid phase bound analytes compete for a fixed number of antibody binding sites. After the system is in equilibrium, free antigen and free antigen-antibody complexes are removed by washing. The antibody bound to the solid phase is detected by an anti-rabbit IgG-peroxidase conjugate using TMB as a substrate. The reaction is monitored at 450 nm. Quantification of unknown samples is achieved by comparing their absorbance with a reference curve prepared with known standard concentrations.The anti-Metanephrine antibodies used in this test kit only recognise the biologically relevant L-forms of Metanephrine. Commercially available synthetic Metanephrine is always a mixture of the D- and L-forms. The ratio between both forms differs widely from lot to lot. This has important implications if synthetic Metanephrine is used to enrich native samples. As only about 50% of the synthetic Metanephrine – the L-portion – will be detected by use of this kit, spiked samples will be underestimated. Therefore native samples containing solely the L-form should be used.Metanephrine and Normetanephrine are the metabolites of the catecholamines Epinephrine and Norepinephrine, respectively. They are metabolized to Vanillylmandelic acid or excreted with the urine. Patients with pheochromocytoma or other tumors derived from neuroendocrine cells show elevated urinary levels of total Metanephrines. As catecholamine secretion from neuroendocrine cells might show high variations, urine samples collected over a period of 24 hours are used to average these fluctuations. Therapeutic consequences should never be based on laboratory results alone even if all test results are in agreement with the items as under point “Procedural cautions, guidelines and warnings”. Any laboratory result is only a part of the total clinical picture of the patient. Only in cases where the laboratory results are in an acceptable agreement with the overall clinical picture of the patient it can be used for therapeutic consequences. The test result itself should never be the sole determinant for deriving any therapeutic consequences.