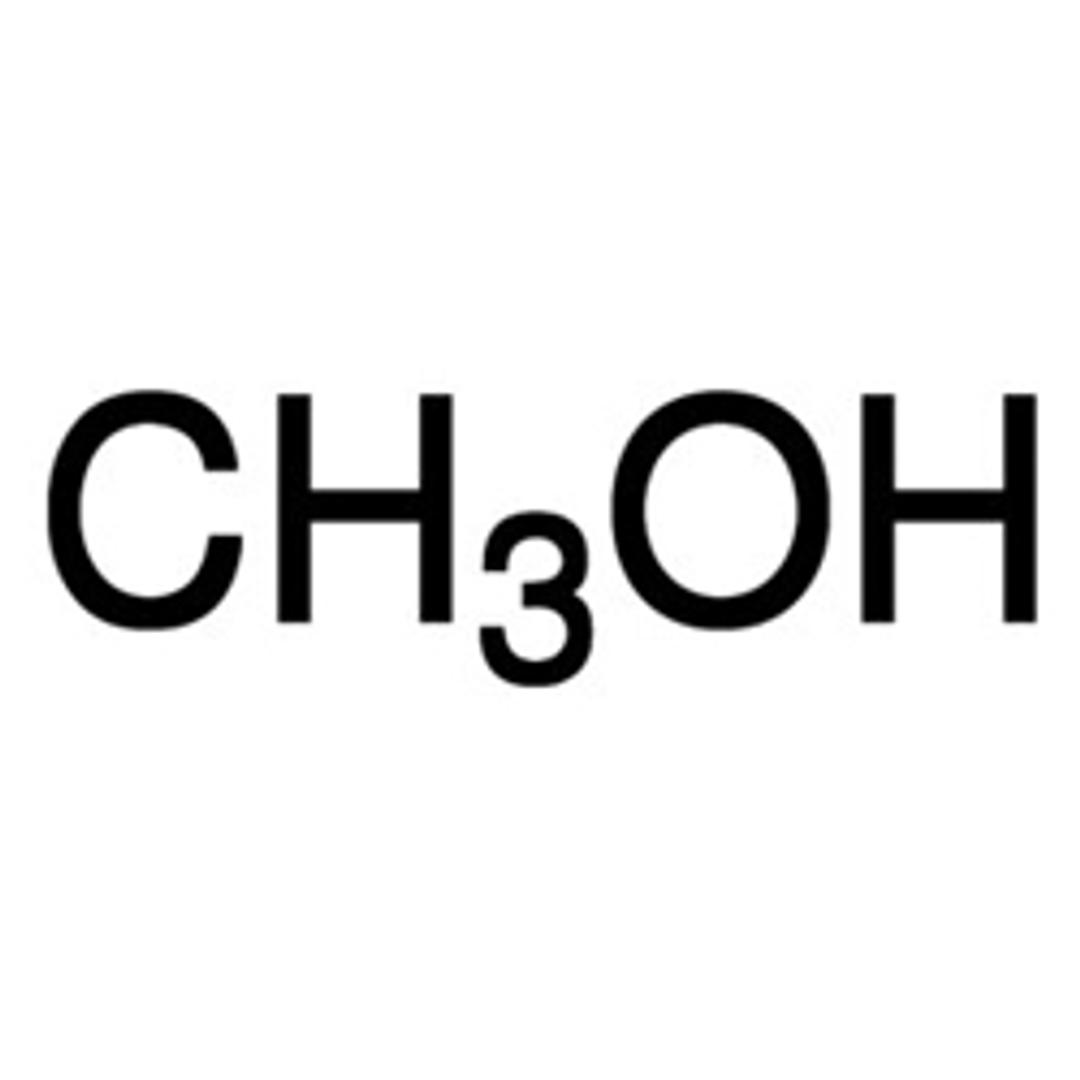
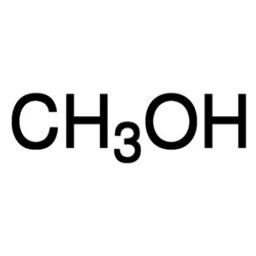
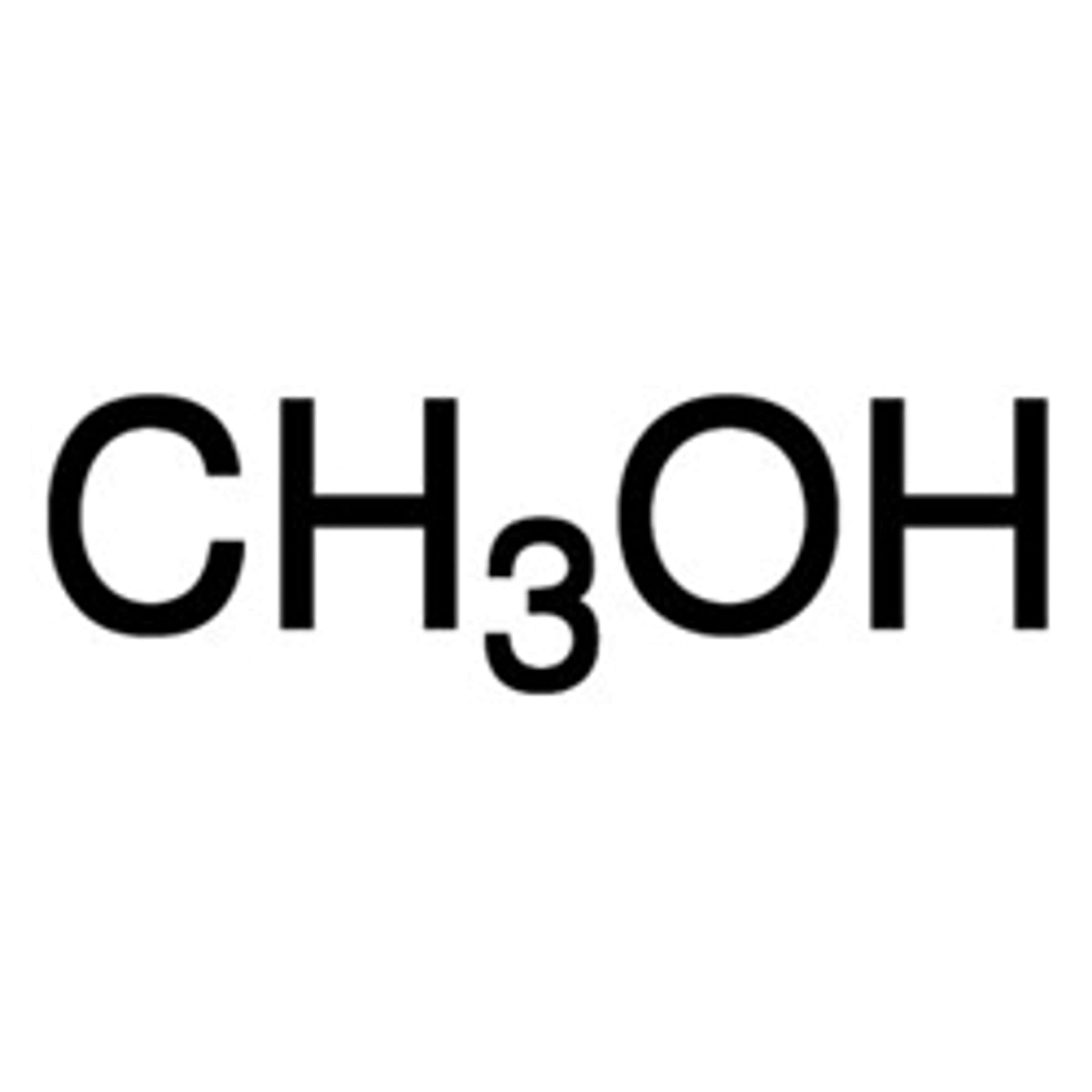
Methanol for HPLC, ≥99.9%
Methanol is an organic solvent that can be synthesized from syngas in the presence of CuO/ZnO/Al2O3 catalysts. It is an ideal candidate as a hydrogen source in fuel cell technology due to its high H/C ratio, low propensity for soot generation, relatively low reforming temperature and as it exists in liquid state at room temperature. In a direct methanol fuel cell (DMFC), methanol undergoes oxidation with air to generate electr…

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
Good product
Extraction
It is so effective
Review Date: 26 Jul 2022 | Sigma-Aldrich Supelco
Quality products from Sigma-Aldrich
Synthesis
Methanol is of high quality (analytical grade), highly recommended for pure synthesis. It is suitable to use for HPLC due to purity as Sigma-Aldrich is a good supplier of chemicals.
Review Date: 3 Dec 2021 | Sigma-Aldrich Supelco
Great ease of use and reproducibility.
Pharmaceutical HPLC
Excellent price and reproducibility.
Review Date: 28 Sept 2021 | Sigma-Aldrich Supelco
Excellent price and reproducibility.
Pharmaceutical HPLC
Excellent price and reproducibility.
Review Date: 28 Sept 2021 | Sigma-Aldrich Supelco
The best methanol you can find for HPLC.
Pharmaceuticals
The purest in this segment and comes with all the documentation required.
Review Date: 2 Feb 2021 | Sigma-Aldrich Supelco
Methanol is an organic solvent that can be synthesized from syngas in the presence of CuO/ZnO/Al2O3 catalysts. It is an ideal candidate as a hydrogen source in fuel cell technology due to its high H/C ratio, low propensity for soot generation, relatively low reforming temperature and as it exists in liquid state at room temperature. In a direct methanol fuel cell (DMFC), methanol undergoes oxidation with air to generate electricity. The olefins (ethylene or propylene) formed from methanol via MTO (methanol-to-olefins) process, can be an alternate to oil and gas to produce hydrocarbon fuels.
Thermochemical conversion of methanol to C2-C10 hydrocarbons in the presence of shape-selective zeolites has been reported. Its oxidation on Ru-Pt catalyst system by ruthenium ad-atoms has been proposed.