QPS Clinical Phase I/IIa Development Services
Moving quickly and safely through Phase I/IIa trials is critical to successful drug development. The QPS Phase I research facilities feature more than 550 beds across six strategically located facilities on three continents. QPS is well known for its success in first-in-human clinical trials. All Phase I sites are staffed by expert clinical pharmacology teams that routinely conduct hundreds of phase I/IIa studies annually.

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
Moving quickly and safely through Phase I/IIa trials is critical to successful drug development.
The QPS Phase I/IIa research facilities feature more than 550 beds across six strategically located facilities on three continents. QPS is well known for its success in first-in-human clinical trials. All Phase I sites are staffed by expert clinical pharmacology teams that routinely conduct hundreds of phase I/IIa studies annually.
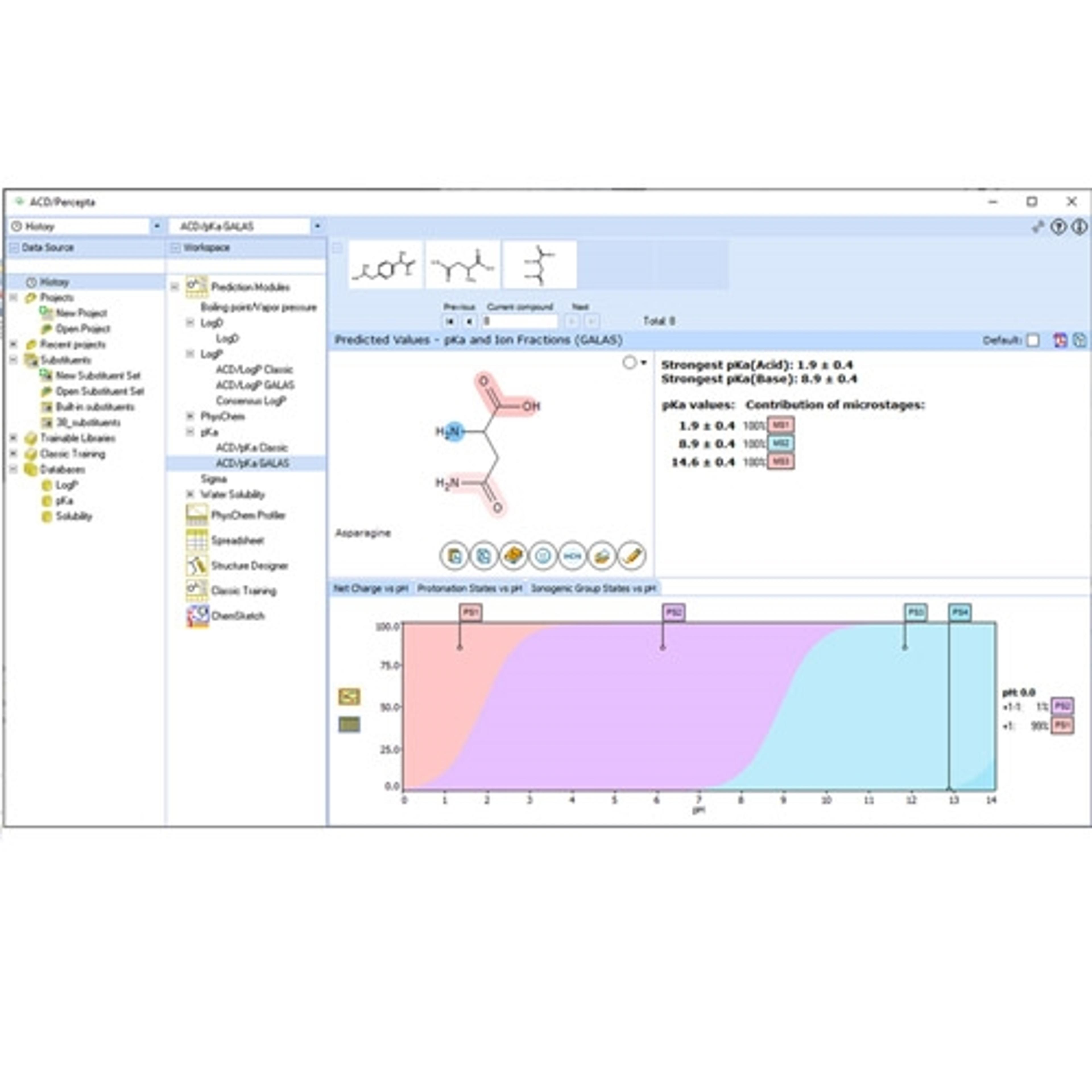
We also have scientific expertise in the design of all types of Phase I studies (formerly referred to as “first-in-man” studies), including dose response, SAD, and MAD studies, as well as the interpretation of study data.
We specialize in conducting proof-of-concept (POC) studies in special and patient populations. Our proof-of-concept studies help ensure a study’s safety and value and prevent resources from being wasted on targets and molecules that aren’t likely to succeed. When well designed and properly conducted, proof-of-concept studies establish the basis for large-scale dose response, efficacy, and safety studies that will assess efficacy, tolerability, side effects, and associated risks.
QPS Global Phase I Facilities:
- 58 beds in Groningen, the Netherlands
- 138 beds in Hyderabad, India
- 8 beds in Leeuwarden, the Netherlands
- 95 beds in South Miami, Florida, USA
- 240 beds in Springfield, Missouri, USA
- 40 beds in Taipei, Taiwan