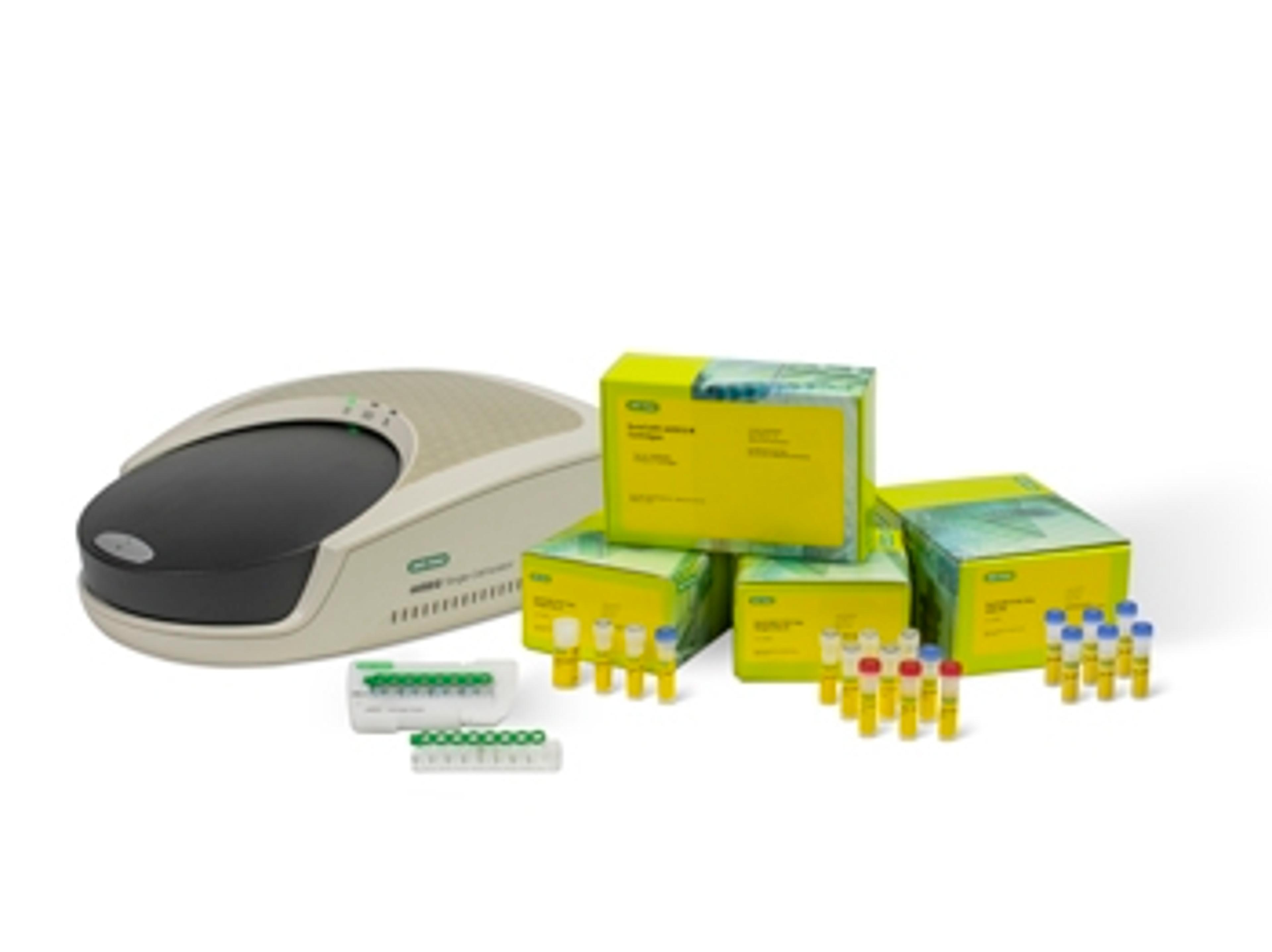
QX ONE™ Droplet Digital™ PCR System
The QX ONE Droplet Digital PCR System integrates droplet generation, thermal cycling, droplet reading, and analysis into a single automated precision platform that delivers superior precision and accuracy and absolute quantification.
Absolutely amazing
Virology
Best to use easy to maintain quality control
Review Date: 15 Jun 2021 | Bio-Rad
Highly useful for clinical management of lung cancer patients!
Oncology
Multiplexing capacity, minimal manual handling, automation in pipetting for droplet generation, has improved the precision and reproducibility of clinical testing results. The technical support team have profound knowledge and they attend to any issue immediately. The sales team and logistics team have been very supportive in expediting the delivery of products to avoid any hurdles in the routine clinical testing of cancer patient samples.
Review Date: 22 Jul 2020 | Bio-Rad
The QX ONE Droplet Digital PCR System integrates droplet generation, thermal cycling, droplet reading, and analysis into a single automated precision platform that delivers superior precision and accuracy and absolute quantification.
WALK-AWAY OPERATION
- Four color channels combined with amplitude multiplexing and/or radial multiplexing allow for multiple unique targets to be detected in a single reaction
- Specially formulated ddPCR Multiplex Supermix is optimized to empower advanced multiplexing in probe-based assays
ADVANCED MULTIPLEXING
- Simple, intuitive run setup using a large onboard touch screen
- Optimized plate setup means fewer handling steps, fewer operator errors, and faster onboarding
EASY TO USE
- QX ONE Software, Regulatory Edition is a robust package that enables U.S. FDA 21 CFR Part 11 compliance and offers audit trails with tracked protocol changes for regulatory requirements
- RFID barcoded consumables allow lot management and traceability
REGULATORY COMPLIANT
- QX ONE Software, Regulatory Edition is a robust package that enables U.S. FDA 21 CFR Part 11 compliance and offers audit trails with tracked protocol changes for regulatory requirements
- RFID barcoded consumables allow lot management and traceability