Gene Therapy Solutions
Svar offers assistance in gene therapy projects through robust platforms suited for all phases of drug development and experience from immunoassasy product development as well as their CRO services. Svar currently offer cell-based assays for Neutralizing antibody assessment and custom-made immunoassays for Total antibody assessment.
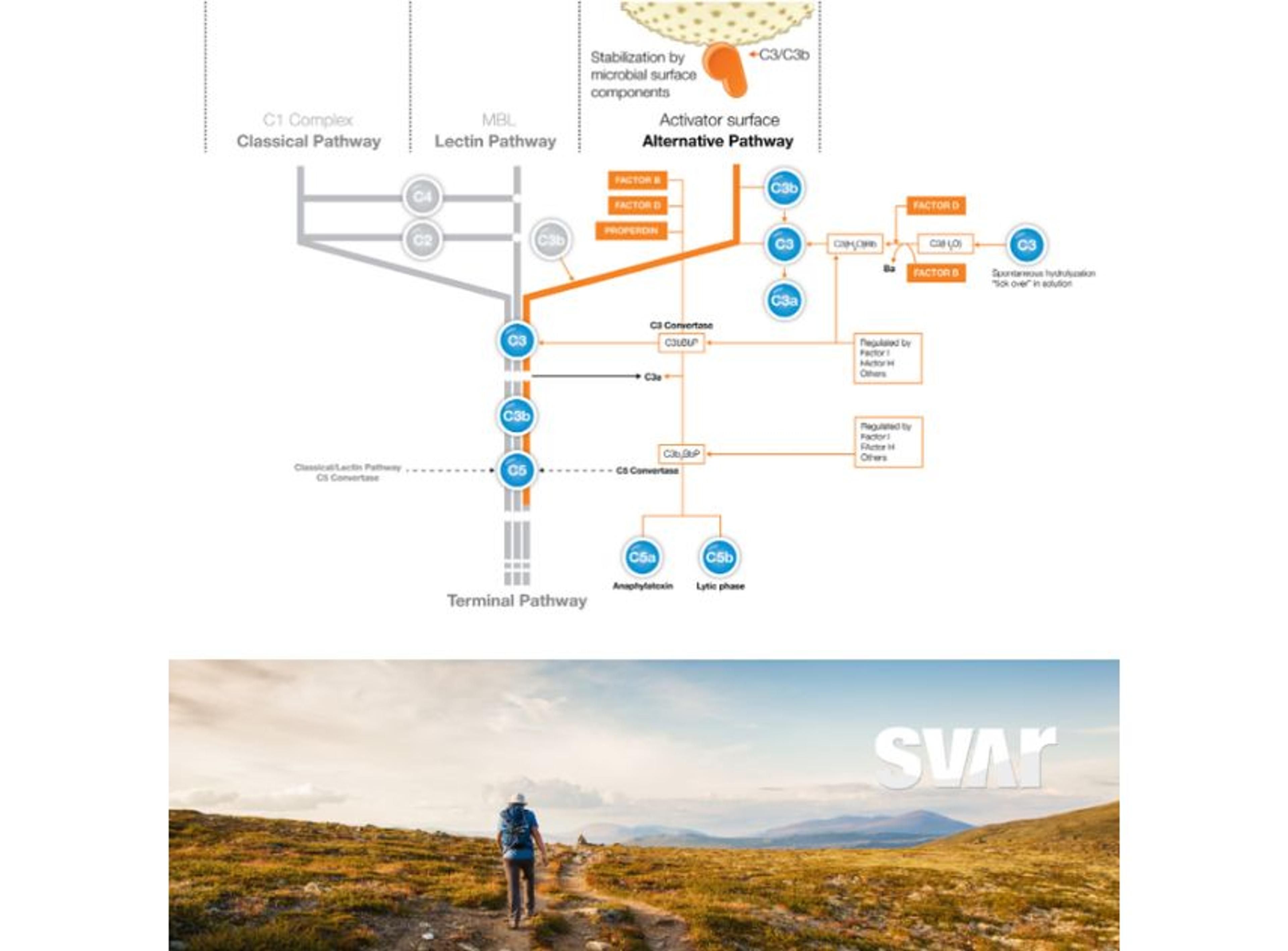
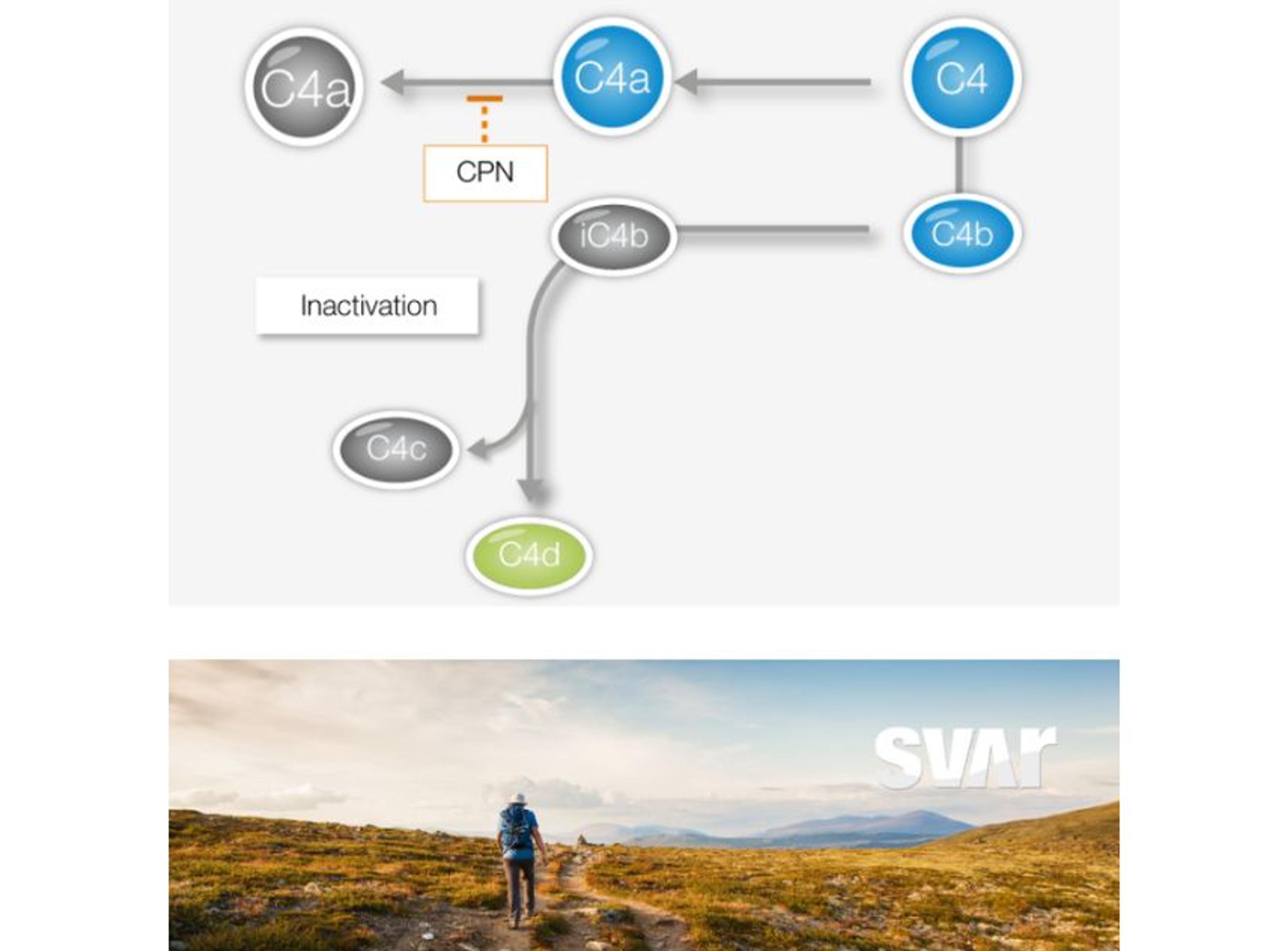
Gene therapy introduces a number of analytical challenges that are not encountered with conventional biopharmaceutical products. Complex and time-consuming bioassays are needed throughout the study to assess product potency, confirm product identity, identify impurities, and test for immunogenicity. With the iLite® Gene therapy solution Svar can offer advanced analytical methods to address these challenges.
Immunogenicity testing
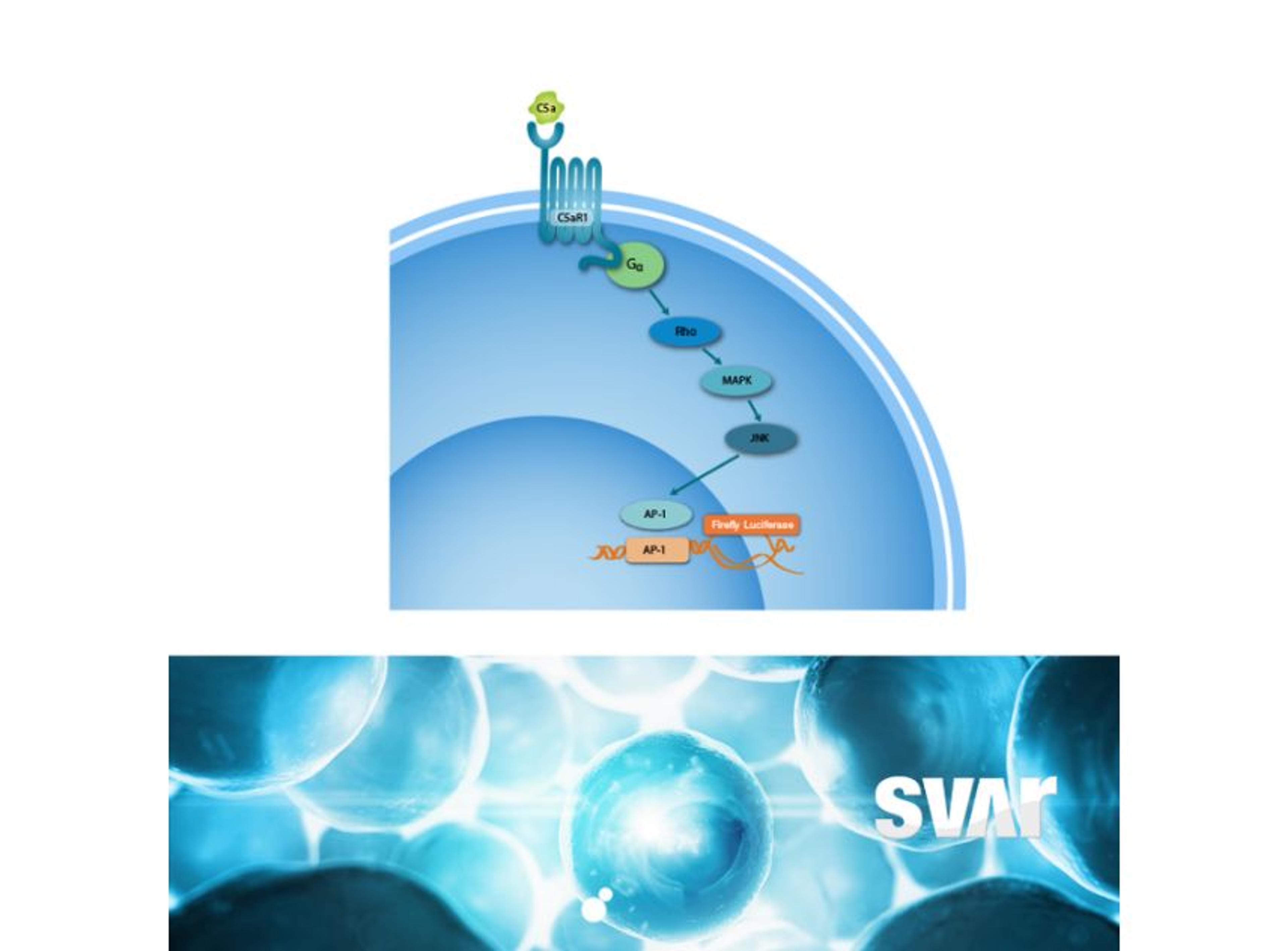
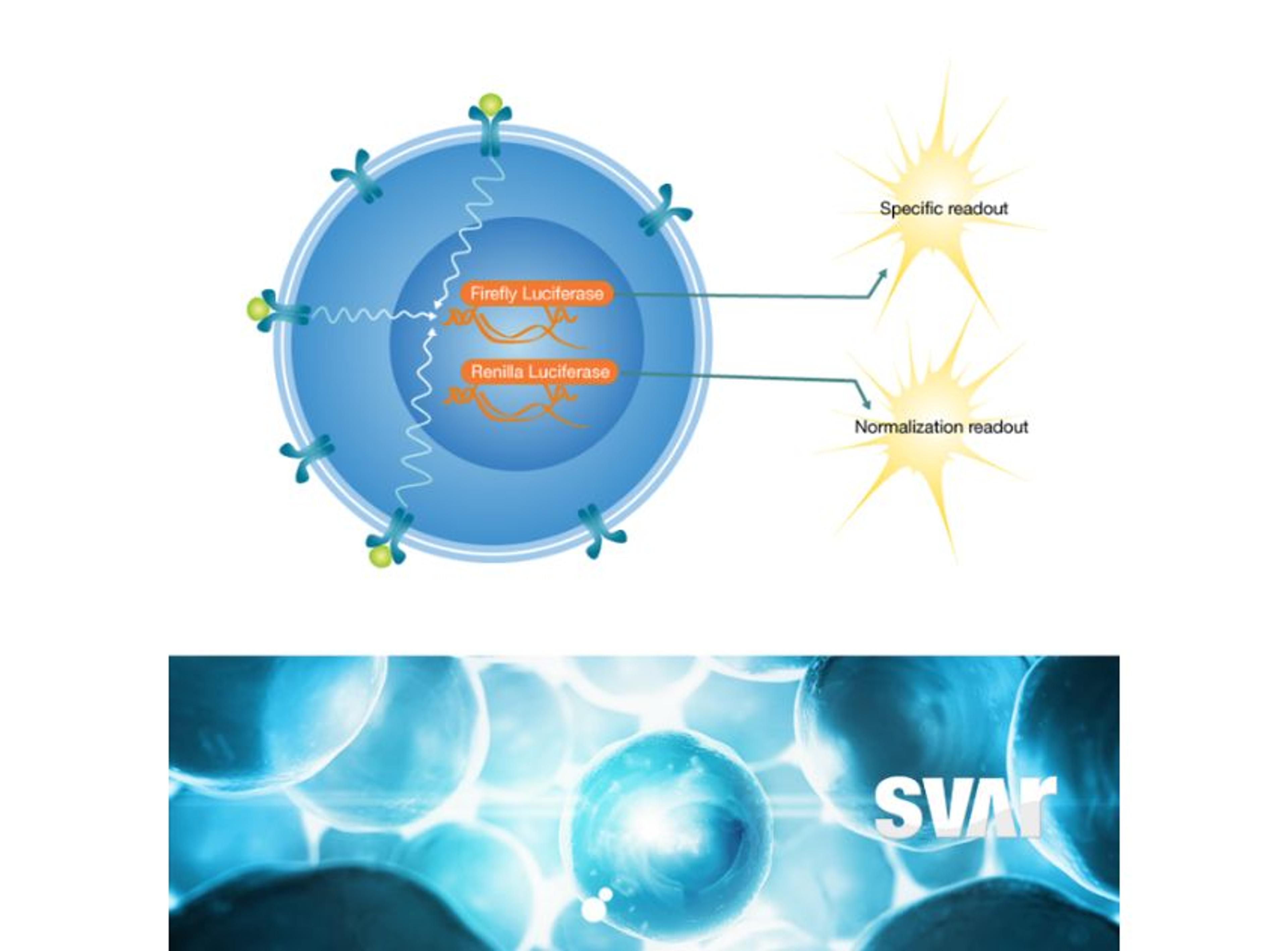
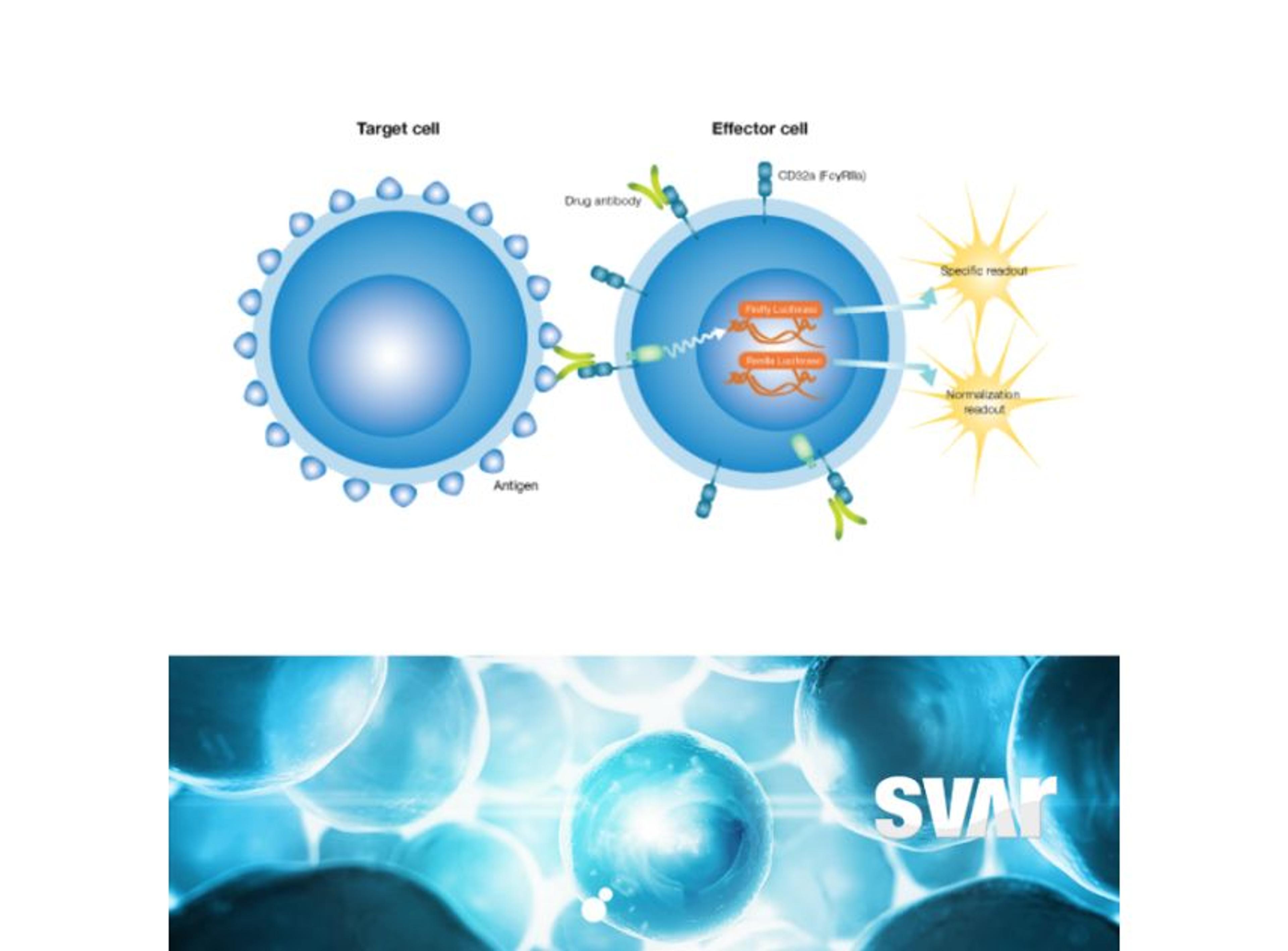
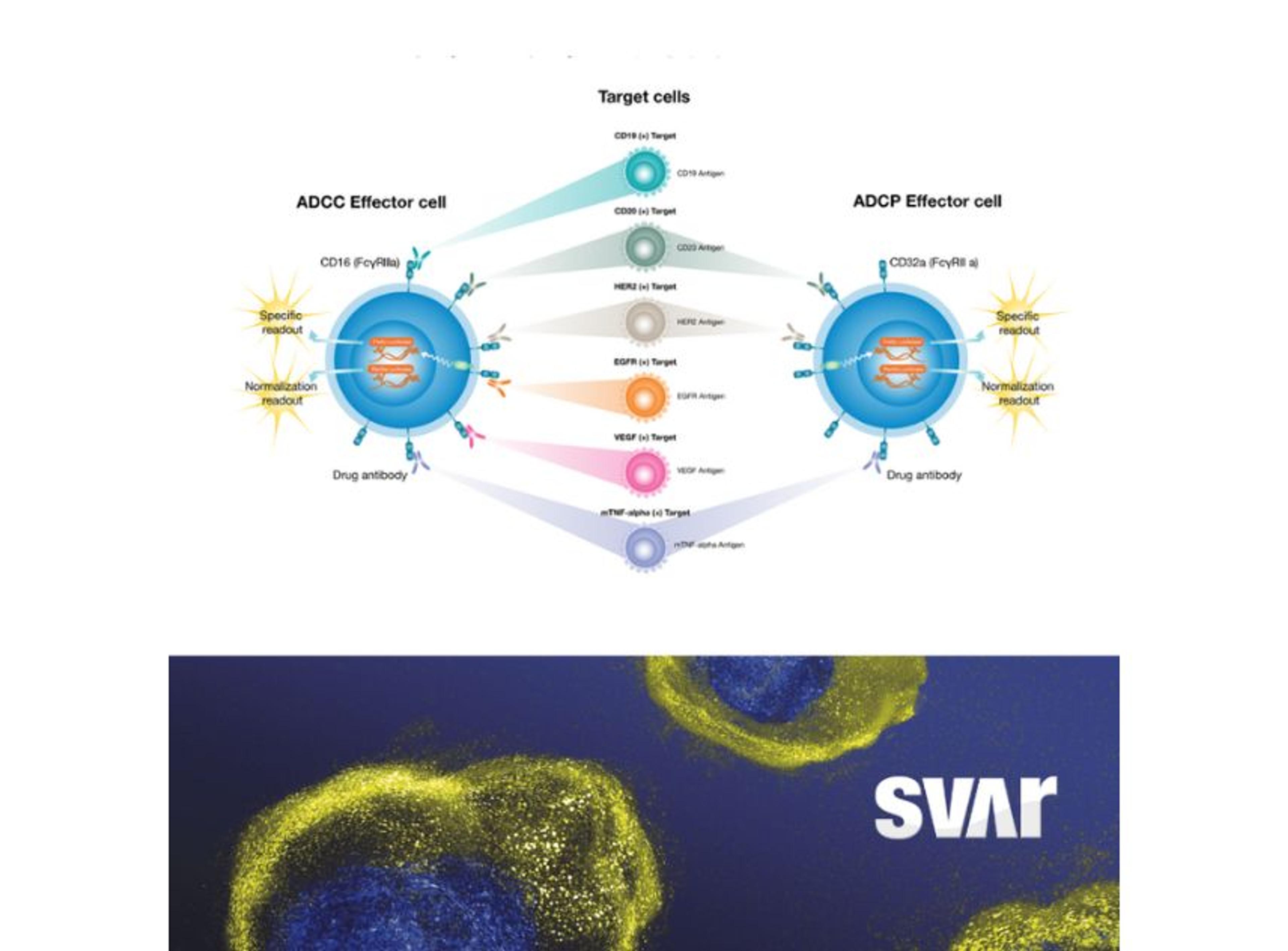
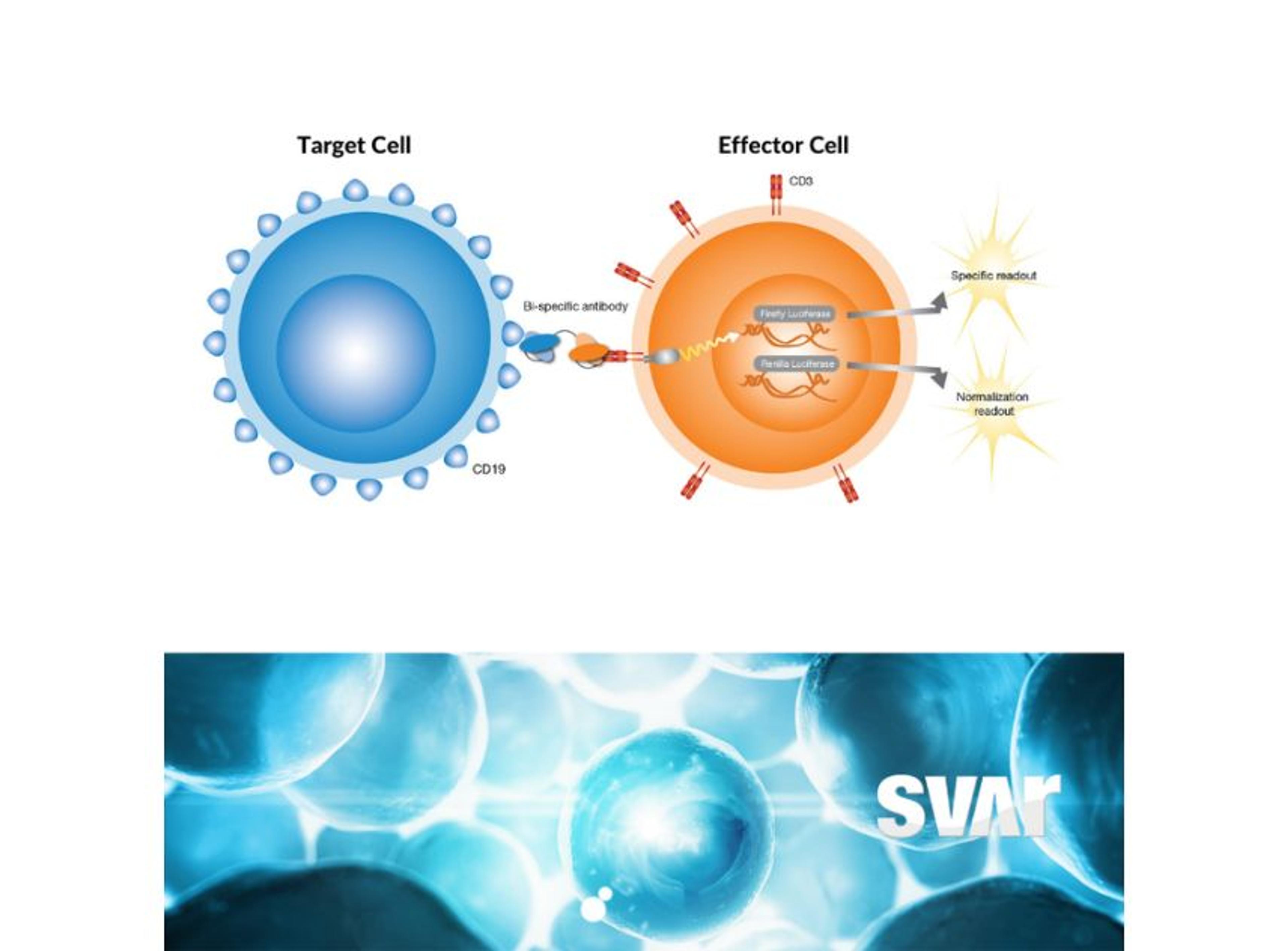
Svar’s iLite® cell-based platform has a long track record of measuring NAbs against biological drugs in patient material. By combining the reproducibility and ease-of-use of assay-ready cells with the robustness of dual reporter gene assays, iLite® assays offer fast and high-quality results for a wide range of targets. These assays are ideal for detecting antibodies against the viral vector in human serum.
Potency testing
Another very important step in gene therapy is analyzing the finished therapeutic product, hence the protein that is being produced by the cells as a result of the corrected or inserted gene. Potency testing is performed in pre-clinical, clinical, or CMC settings to show a clinical effect and ensure batch-to-batch consistency. This is another area where iLite® technology is ideally suited. Svar Life Science is developing a robust in vitro assay for the measurement of therapeutic product potency.
Svar Bioanalytical Services
Gene therapy is an advanced analytical field with many new types of challenges. Svar's Bioanlaytical services have already gained experience from such projects. Svar Life Science has, among other things, performed immunogenicity projects for investigating antibody response against the gene therapy product and measured the protein produced from the gene product. Furthermore, Svar Life Science has developed a TK assay for a MAb drug that will be administered as a gene product in non-clinical and clinical studies. Svar's goal is to leverage the knowledge from their customized gene therapy projects into their service offering and delivering bioanalytical excellence through a collaborative approach.