Veterinary Drug PCDL for LC/TOF and LC/Q-TOF
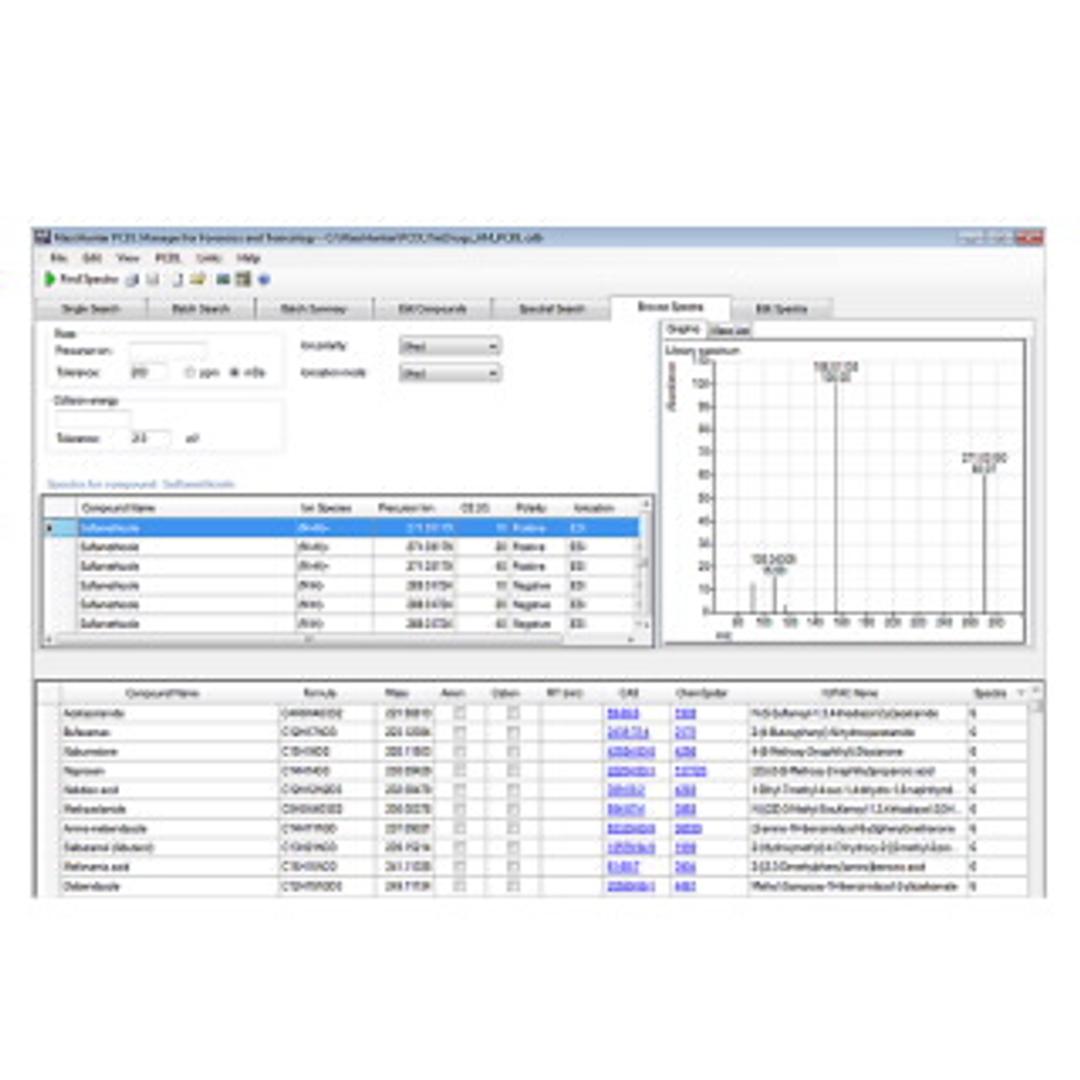
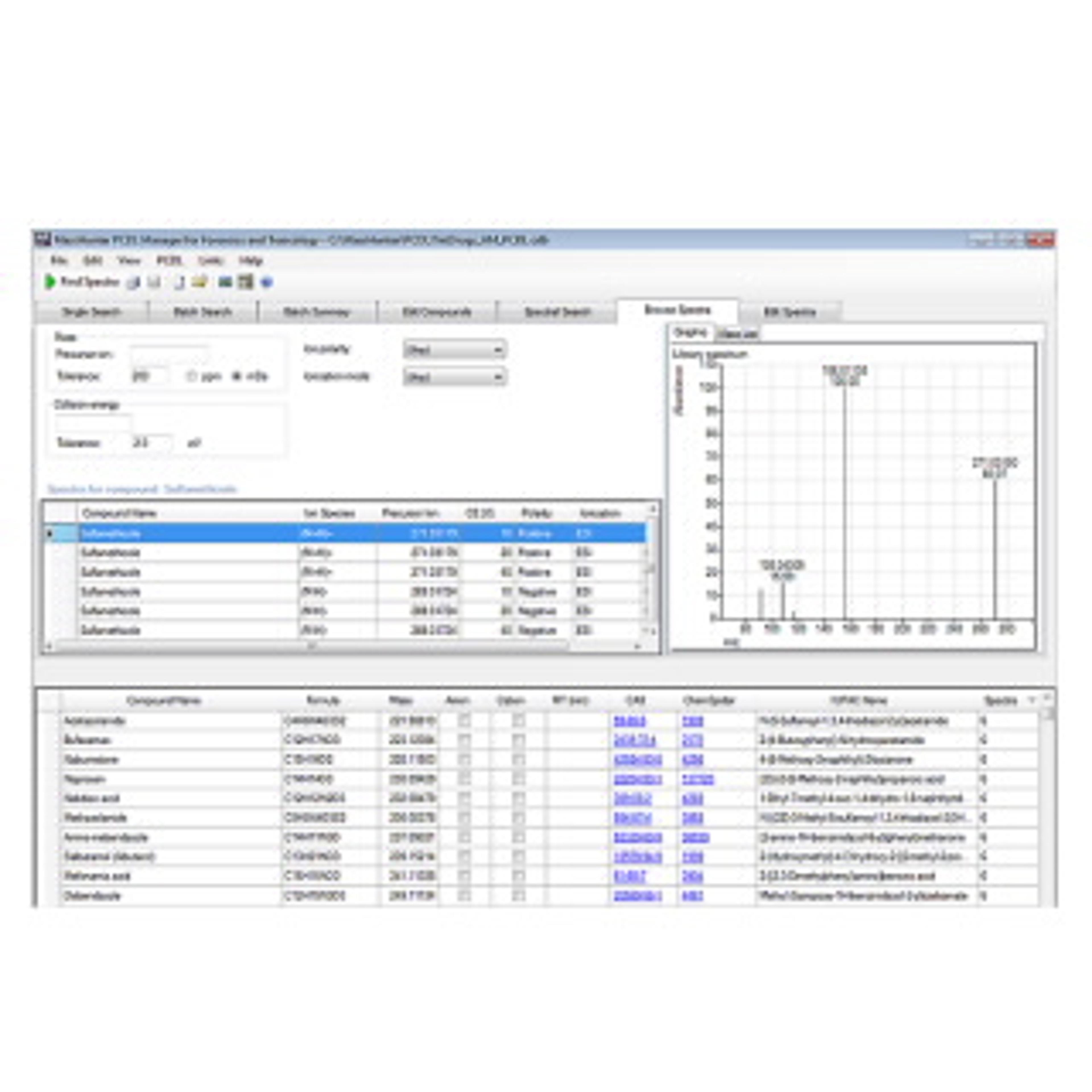
The Veterinary Drug Personal Compound Database and Library (PCDL) for TOF or Q-TOF LC/MS systems contains a curated accurate-mass database with over 2,100 compounds, accurate-mass MS/MS spectra for more than 1,500 compounds, and retention time information for 120 compounds. Detailed acquisition method setup information provided allows for fast ramp-up to full productivity.
The Veterinary Drug Personal Compound Database and Library (PCDL) for TOF or Q-TOF LC/MS systems contains a curated accurate-mass database with over 2,100 compounds, accurate-mass MS/MS spectra for more than 1,500 compounds, and retention time information for 120 compounds. Detailed acquisition method setup information provided allows for fast ramp-up to full productivity.
Features:
- Enjoy seamless integration of the PCDL into Agilent MassHunter Qualitative software for simplified screening workflows.
- Acquire full-spectrum, untargeted data using All Ions MS/MS and identify compounds through accurate mass, retention time, isotope pattern, and fragment confirmation.
- Perform presumptive matching of acquired spectra with library spectra–without the need to source standards.
- Create a custom PCDL from the Agilent PCDL for a more focused screening approach, or add your own unique compounds and library spectra to create PCDLs specific to your analysis.
- Propose a suspect list with MS-only data, then confirm compound presence and eliminate false positives with targeted MS/MS and library search.
- Mine data from Auto MS/MS experiments and search for proposed compounds against the PCDL
- Perform retrospective analysis of data with compounds newly added to the PCDL, without a need to re-run samples.
- Keep your screening experiments current with free PCDL updates for 3 years.
- Get best in class application services and consumables tailored to your lab from Agilent CrossLab.
- Compatible with all current models of Agilent TOF or Q-TOF LC/MS systems.